Influenza vaccine composition for sublingual mucosa delivery
A technology of influenza vaccine and composition, which is applied in the direction of microorganisms, biochemical equipment and methods, and medical preparations containing active ingredients, etc., which can solve the problems of insufficient immunogenicity, immune tolerance of SLIT administration, and induction of immune tolerance and other problems, to achieve the effect of fast and convenient use, benefiting large-scale immunization, and improving immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of Example 1 Influenza Vaccine Sublingual Rapidly Disintegrating Tablet
[0032] In order to facilitate the screening, the present invention makes the influenza vaccine composition into sublingual rapidly disintegrating tablets, and investigates the influencing factors on the immunogenicity and immune tolerance of the influenza vaccine. It should be noted that the focus of the present invention is to explore the relationship between the screening of adjuvants on the immunogenicity and immune tolerance of influenza vaccines, not to investigate the immunogenicity and immune tolerance of influenza vaccines by changing the application dosage form of the influenza vaccine composition. influence relationship. Therefore, the sublingual rapidly disintegrating tablet of influenza vaccine prepared in Example 1 is only an example of SLIT administration, but the dosage form application of the influenza vaccine composition is not limited to the sublingual rapidly disinte...
Embodiment 2
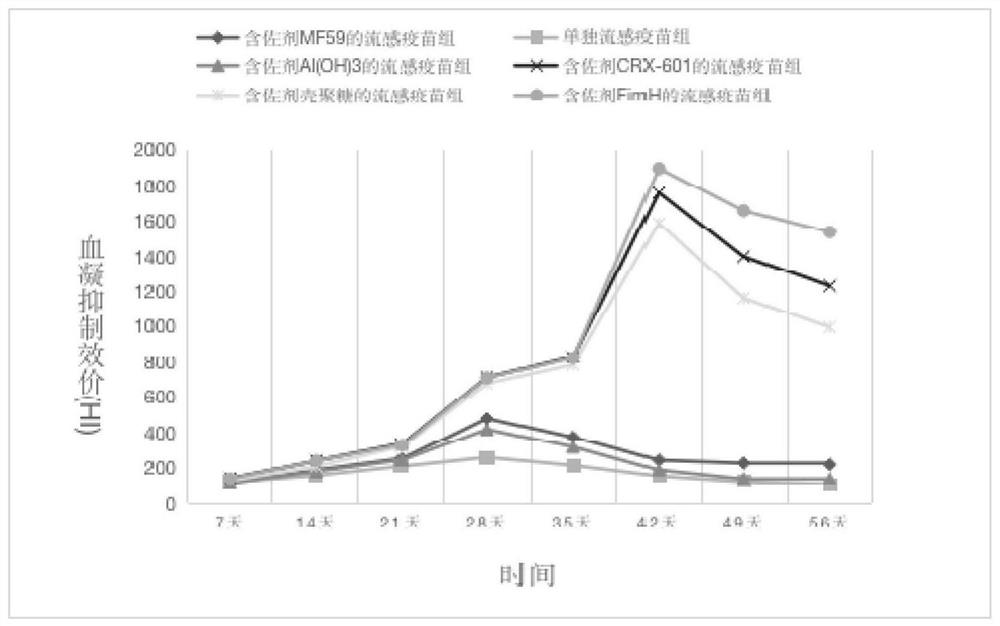
[0036] The impact of the kind of embodiment 2 adjuvants on the immunogenicity of influenza vaccine
[0037] The purpose of this example is to investigate the impact of the type of adjuvant on the immunogenicity of influenza vaccine, and select FimH adjuvant, MF59 adjuvant, CRX-601 adjuvant, chitosan adjuvant, and aluminum hydroxide adjuvant for comparative research.
[0038] The inspection method is as follows:
[0039] 1) Take the prescribed amount of FimH adjuvant, MF59 adjuvant, CRX-601 adjuvant, chitosan adjuvant, aluminum hydroxide adjuvant, and set aside;
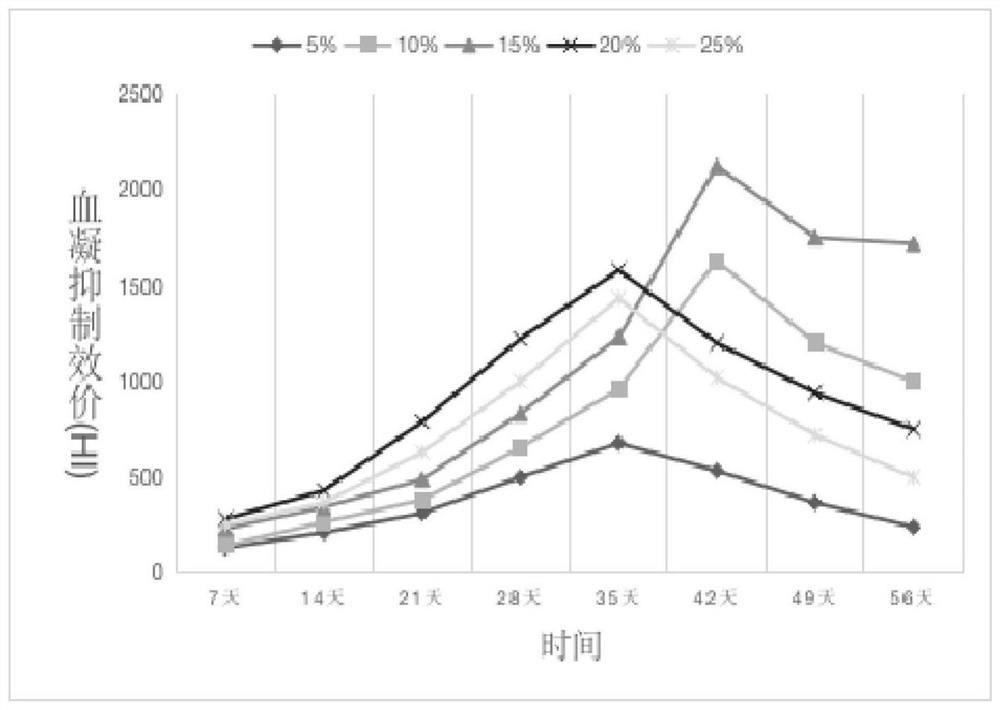
[0040] 2) Fix the component ratio of the adjuvanted influenza vaccine to the tablet matrix material, refer to (Huang Jihan, Huang Xiaohui, Chen Zhiyang, et al. Equivalent dose conversion between animals and between animals and humans in pharmacological tests[J]. China Clinical Pharmacology and Therapeutics, 2004, 9 (9): 1069-1072.) article, the dosage of human sublingual tablet is converted into the dosage of mouse s...
Embodiment 3
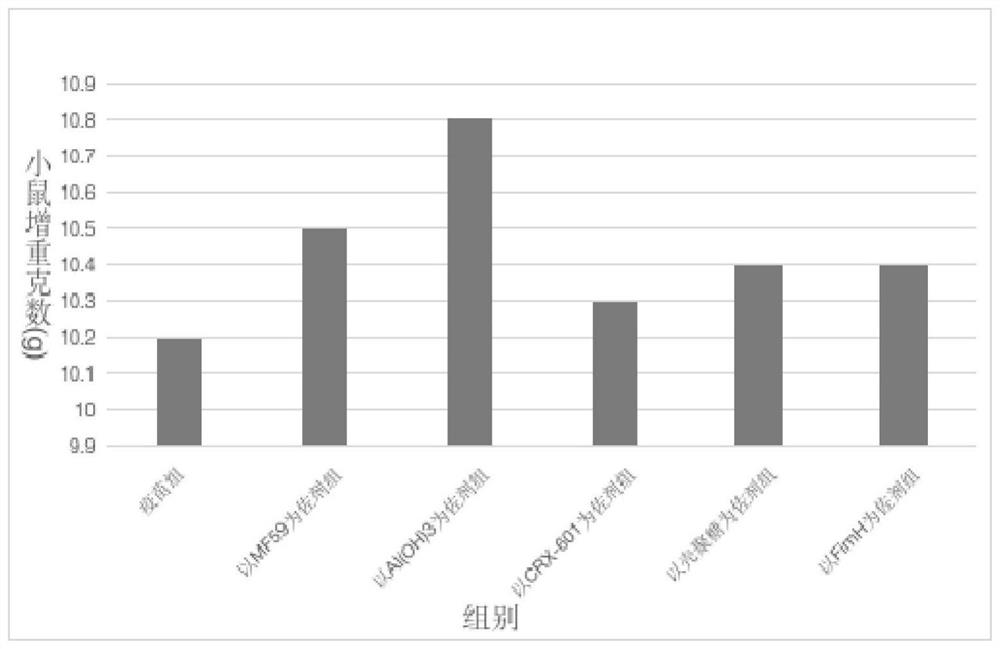
[0048] Example 3 The impact of the type of adjuvant on sublingual mucosal immune tolerance
[0049] The purpose of this example is to investigate the impact of the type of adjuvant on sublingual mucosal immune tolerance, and select FimH adjuvant, MF59 adjuvant, CRX-601 adjuvant, chitosan adjuvant, and aluminum hydroxide adjuvant for comparative research .
[0050] The inspection method is as follows:
[0051] 1) Take the prescribed amount of FimH adjuvant, MF59 adjuvant, CRX-601 adjuvant, chitosan adjuvant, aluminum hydroxide adjuvant, and set aside;
[0052] 2) Fix the component ratio of the adjuvanted influenza vaccine and the tablet matrix material, and prepare the corresponding sublingual rapidly disintegrating tablet of influenza vaccine according to the preparation method in Example 2. Mice were inoculated with SLIT. The sublingual mucosa was isolated at 0 or 2 hours after SLIT immunization, and cells were harvested from the mucosa and analyzed by flow cytometry. Two...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com