Mycoplasma hyopneumoniae and haemophilus parasuis bivalent pentavalent inactivated vaccine and application thereof
A technology of Mycoplasma hyopneumoniae and Haemophilus suis applied in the direction of multivalent vaccines, vaccines, veterinary vaccines, etc., can solve the problems affecting the healthy development of the pig industry, economic losses of the pig industry, and lack of immune cross-protection ability. Good immunogenicity, good immune effect, good cross-protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Isolation and identification of Mycoplasma hyopneumoniae
[0027] 1.1 Collection of disease data
[0028] The disease material of Mycoplasma hyopneumoniae came from the lungs of a 2-month-old nursery pig that had severe pneumonia and dyspnea in a large pig farm in Puyang City, Henan Province in April 2017.
[0029] 1.2 Culture medium preparation
[0030]PPLO liquid medium: 10.5 g of PPLO broth powder, 2.5 g of glucose, and 2.5 g of yeast powder, dissolved in 440 mL of ultrapure water, sterilized at 115°C for 15 min, added with 5 mL of MEM medium, 50 mL of horse serum, penicillin 80,000 units, 10 mL of sterile 10% arginine and 500 μL of 1% (w / v) phenol red. After the culture was sterilized at 115°C for 15 minutes, it was stored at 4°C for later use.
[0031] PPLO solid medium: PPLO liquid medium with 1.5% (w / v) agar powder added. After the culture was sterilized at 115°C for 15 minutes, it was stored at 4°C for later use.
[0032] 1.3 Isolation and cul...
Embodiment 2
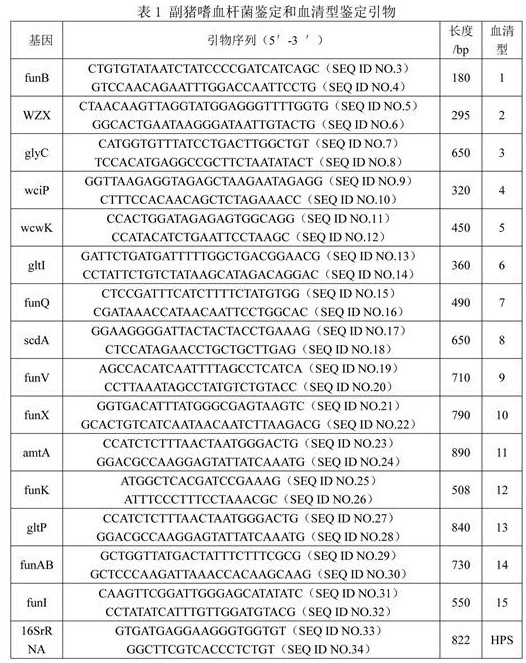
[0047] Embodiment 2: Isolation and identification of Haemophilus parasuis
[0048] 2.1 Sources of disease materials
[0049] From January 2016 to December 2019, a total of lung, heart blood, lymph node, Spleen, brain, joint fluid and other disease materials, the diseased pigs often manifested as fever, dyspnea, joint swelling, lameness, cyanosis of the skin and mucous membranes, difficulty in standing or even paralysis, stiff pigs or death.
[0050] 2.2 Culture medium preparation
[0051] TSB liquid medium: 30 g of TSB broth powder (Tryptic Soy broth, tryptone soybean broth, BD Company) was dissolved in 1000 mL of ultrapure water, sterilized at 115 °C for 15 min, and 50 mL of fetal bovine serum (Hychone Company), sterile 1% NAD (Nicotinamide adenine dinucleotide, coenzyme Ⅰ, Roche Company) 1mL.
[0052] TSA solid medium: Dissolve 40 g of TSA agar powder (Tryptic Soy Agar, Tryptone Soy Agar, BD Company) in 1000 mL of ultrapure water, sterilize at 115 ° C for 15 min, add 50 m...
Embodiment 3
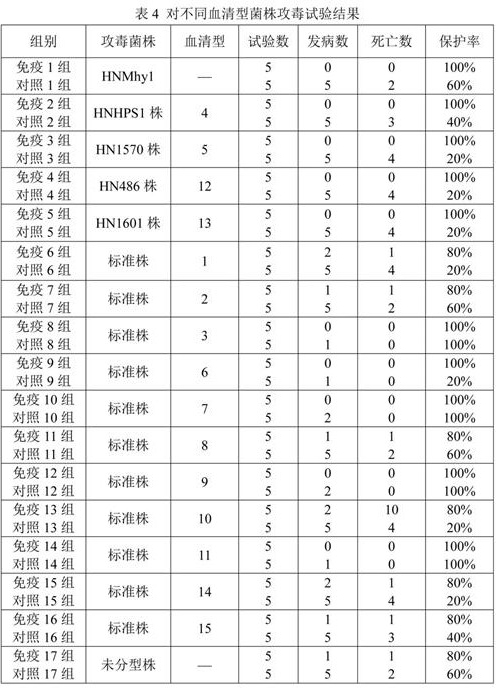
[0072] Embodiment 3: Preparation, safety and efficacy test of vaccine
[0073] 3.1 Preparation of the vaccine
[0074] 3.1.1 Breeding of strains and strains
[0075] Primary Seed Propagation
[0076] The Mycoplasma hyopneumoniae HNMhy1 strain was inoculated on PPLO solid medium at 37°C, 5% CO 2 Cultured in an incubator, harvested after 3 days.
[0077] The freeze-dried strains of Haemophilus parasuis type 4 HNHPS1 strain, type 5 HN1570 strain, type 12 HN486 strain and type 13 HN1601 strain were respectively inoculated in the culture medium containing newborn bovine serum (final concentration 5%) and NAD (final concentration 0.01%). On the TSA plate, cultivate at 37°C for 18-24h, pick typical colonies that meet the requirements, subculture and inoculate the TSA plate containing newborn bovine serum (final concentration 5%) and NAD (final concentration 0.01%), and culture at 37°C for 24h. After passing the inspection, it will be regarded as the first-class seed.
[0078] se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com