Tubular magnetic particle chemiluminescence immunoassay quantitative kit for detecting CD47 as well as preparation method and application of tubular magnetic particle chemiluminescence immunoassay quantitative kit
A chemiluminescence immunoassay and CD47 technology, applied in the field of immunoassay medicine, can solve the problem of less research on the in vitro detection method of CD47 protein, and achieve the effects of expanding the area of the solid phase carrier, sufficient signal amplification, rapid enrichment and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
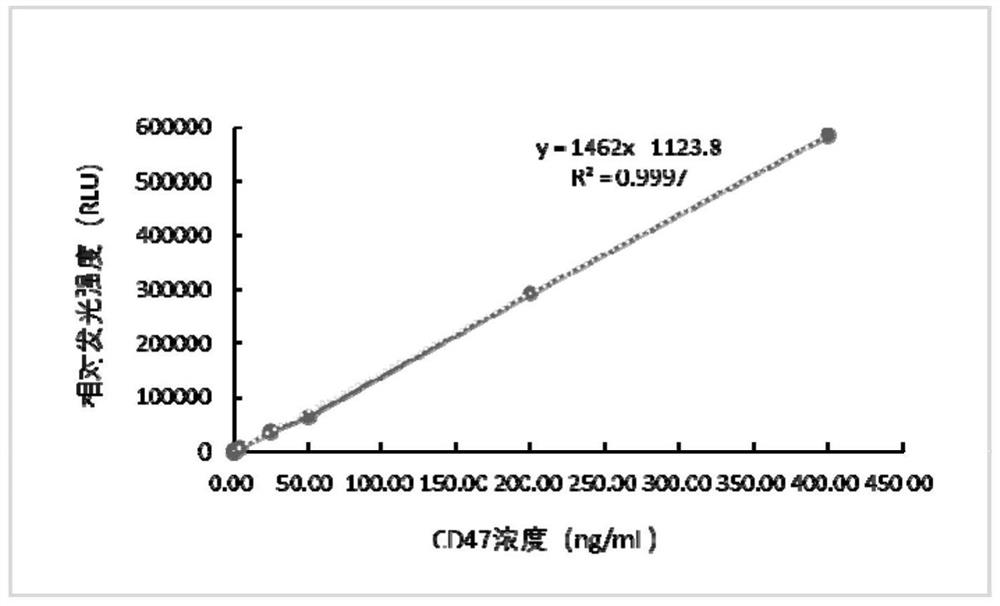
[0054] A tubular magnetic particle chemiluminescence immunoassay kit for detecting CD47, including magnetic particle complex, sample diluent, enzyme conjugate, luminescent liquid A, luminescent liquid B, 20× concentrated detergent, CD47 standard gradient solution, Reaction tube and instruction manual;
[0055] Wherein, the magnetic particle complex is a magnetic particle-immunoglobulin binding molecule-CD47 antibody complex; the enzyme conjugate is a CD47 antibody-neutravidin-biotin-horseradish peroxidase complex The CD47 antibody is a biotin-labeled CD47 monoclonal antibody; the immunoglobulin binding molecule is staphylococcal protein A.
[0056] The formula of the sample diluent is: sterilized 1L purified water containing 8g of NaCl, NaH 2 PO 4 2H 2 O 0.2g, NaH 2 PO 4 2H 2 O 2.5g, casein sodium salt 3.5g, Proclin300 1.0mL.
[0057] The luminescent liquid A contains luminol 4mmol / L, p-iodophenol 0.5mmol / L, FeCl 3 15μmol / L, dioxane 3mL / L, Tris-HCl buffer 5mmol / L, pH ...
Embodiment 2
[0066] A method for preparing a tubular magnetic particle chemiluminescence immunoassay kit for detecting CD47, comprising the following steps:
[0067] S1: Preparation of magnetic particle-immunoglobulin binding molecule-CD47 antibody complex
[0068] Place 500 μg of carboxyl magnetic particles in MES (50 mM, pH 5) buffer, then add 120 μg of EDC and 120 μg of Sulfo-NHS, incubate with shaking at room temperature for 45 min, centrifuge and wash, add 120 μg of staphylococcal protein A and incubate for 3.5 h, then Wash by centrifugation, add 120 μg of CD47 monoclonal antibody and incubate for 3.5 hours with the magnetic particle-immunoglobulin binding molecule to obtain the magnetic particle-immunoglobulin binding molecule-CD47 antibody complex;
[0069] S2: Preparation of CD47 antibody-neutravidin-biotin-horseradish peroxidase complex
[0070] 1) Preparation of CD47 antibody-neutravidin
[0071]Dissolve 40 mg of neutravidin in 100 mL of 0.1 mol / L phosphate buffer, add 0.6 mL o...
Embodiment 3
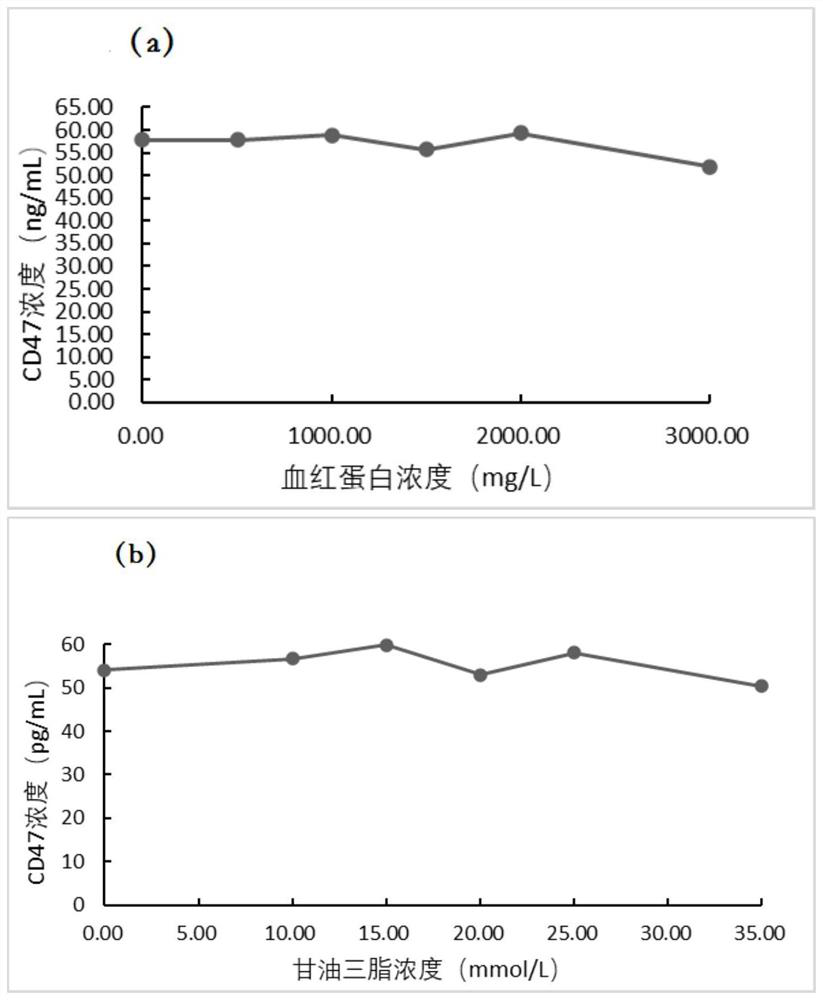
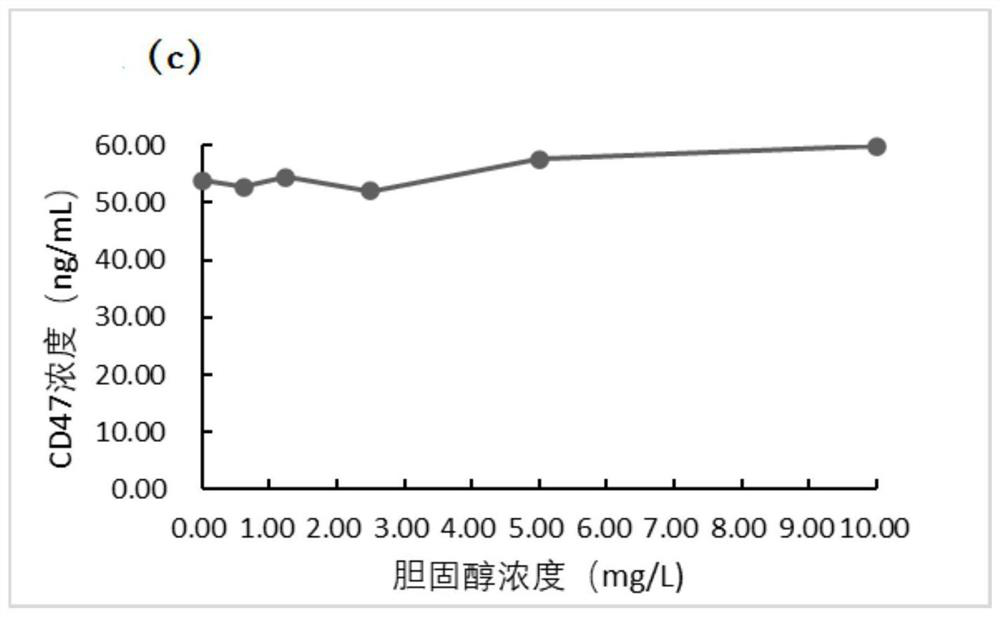
[0079] The application of the tubular magnetic particle chemiluminescence immunoassay kit described in the embodiment in the detection of CD47, the detection method is as follows:
[0080] (1) Equilibrate the sample to be tested and the reagents in the kit at 20-25°C for 25±5min, dilute the washing solution 20 times with distilled water and set aside;
[0081] (2) Set standard wells and sample wells to be tested, add 50 μL magnetic particle complex suspension to the sample wells to be tested and standard wells respectively, then add 50 μL sample to be tested and 50 μL CD47 standard substance gradient solution respectively, and then Add 50 μL of sample diluent, then incubate at 37°C and shake for 10 minutes;
[0082] (3) Place the reaction tube on the magnetic separator and let it stand for 10 seconds, discard the liquid, remove the reaction tube from the magnetic separator, rinse the magnetic particle complex with detergent, perform magnetic separation after washing, and disca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com