Thiophene polymer film as well as preparation method and application thereof
A polymer film, polymer technology, used in chemical instruments and methods, color-changing fluorescent materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] Preparation of E2SBFE2 monomer:
[0025] (1) Add 20 grams of 3,4-ethylenedioxythiophene to 500 milliliters of ultra-dry dichloromethane under a nitrogen atmosphere, and place it at 0°C, and add 70 milliliters of 2.4 mol / L of n-butyllithium n-hexane solution, continue to stir for 1 hour, then add 50 grams of tributyltin chloride dropwise therein, and after stirring for 1 hour, take it out from the 0°C environment and place it at room temperature to continue stirring for 12 Hour. Add 600mL of dichloromethane and 600mL saturated sodium chloride solution to the obtained 600mL solution after the reaction, extract 3 times, combine the obtained organic phases, add 20g of anhydrous sodium sulfate therein and stir for 30 minutes to dry, and then extract Filter to remove sodium sulfate therein, add 40g of silica gel powder to the dried organic phase and remove the organic solution using a rotary evaporator, the resulting crude product is separated and purified by chromatographi...
Embodiment 1
[0030] Dissolve 0.2g of E2SBFE2 and 0.4g of supporting electrolyte tetrabutylammonium hexafluorophosphate in 100ml of chromatographic grade methylene chloride to obtain the required electrolyte for electrochemical polymerization. Electrochemical polymerization adopts a three-electrode system, with indium tin oxide glass (ITO) as the working electrode, platinum sheet as the counter electrode, and silver / silver chloride as the reference electrode. Polymerization was carried out at room temperature with a constant potential method at a voltage of 1.4V and an electric quantity of 0.05C. After the polymerization was completed, the electrolyte solution was replaced with a 4.0 g / L tetrabutylammonium hexafluorophosphate dichloromethane solution, and a constant potential method was used to dedope at -0.2 V for 60 s to obtain a P(E2SBFE2) film.
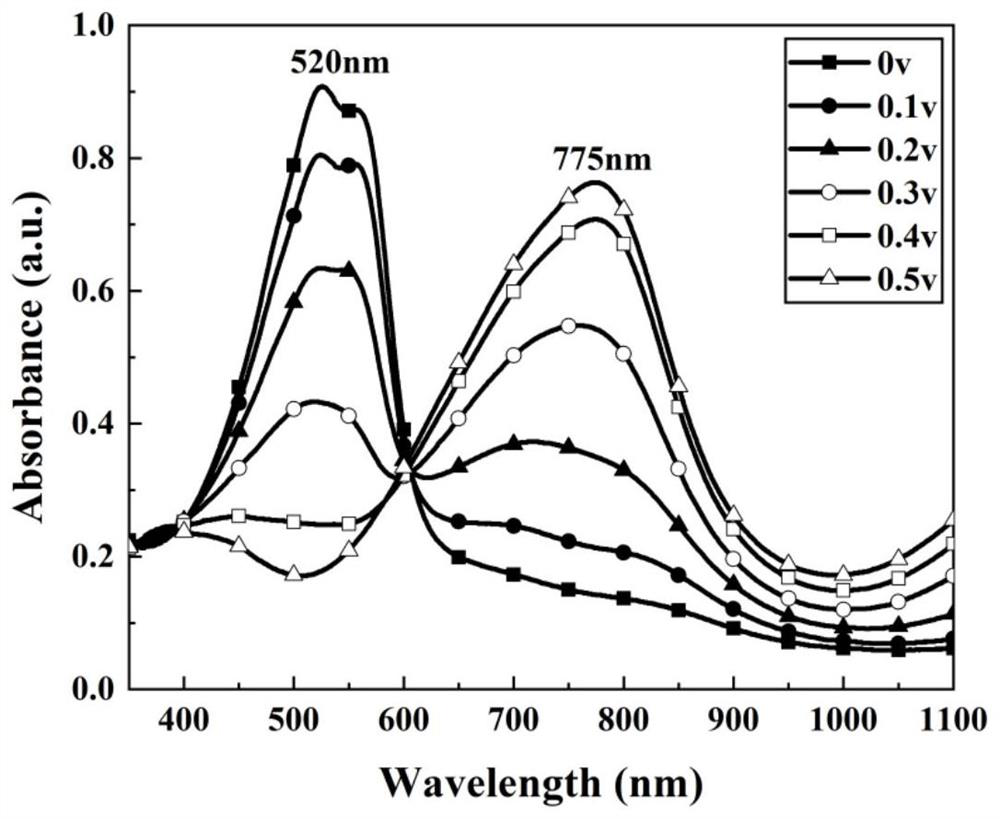
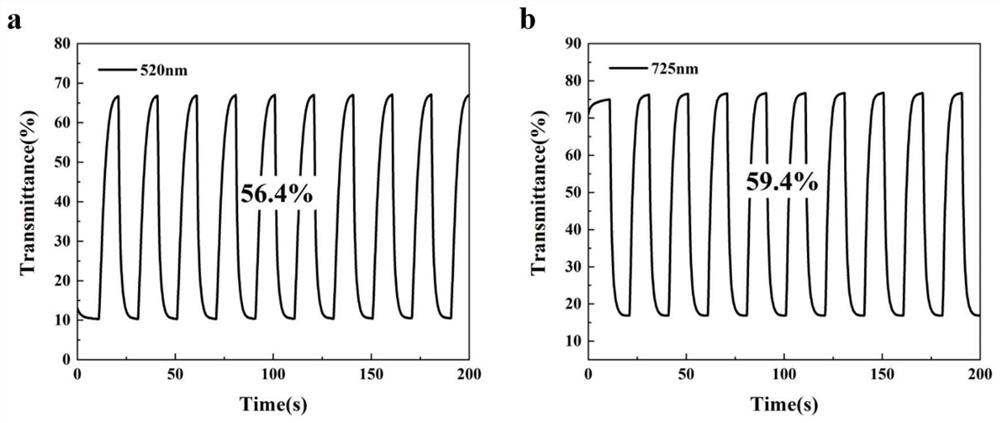
[0031] The P (E2SBFE2) thin film that is loaded on the ITO glass that embodiment 1 prepares is carried out scanning electron microscope analys...
Embodiment 2
[0039] Dissolve 0.2g of E2SBFE2 and 0.4g of supporting electrolyte tetrabutylammonium hexafluorophosphate in 100ml of electrolytic solvent chromatographic grade methylene chloride to obtain the electrolytic solution required for electrochemical polymerization. Electrochemical polymerization adopts a three-electrode system, with indium tin oxide glass (ITO) as the working electrode, platinum sheet as the counter electrode, and silver / silver chloride as the reference electrode. Polymerization was carried out by cyclic voltammetry at room temperature, with forward scanning from 0V to 1.4V, the scanning speed was 100mV / s, and the number of scanning cycles was 5 cycles. After scanning, the electrolyte was replaced with 4.0g / L tetrabutylammonium hexafluorophosphate chromatographic grade dichloromethane solution, and the constant potential method was used to dedope at -0.2V for 60s to obtain a P(E2SBFE2) film.
[0040] The P (E2SBFE2) thin film that the load of embodiment 2 is prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com