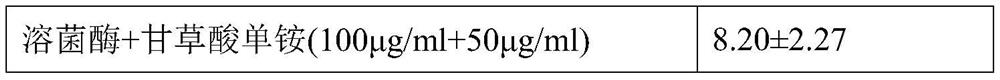Medicine and food for resisting new corona-virus infection and application
A virus infection and virus infection-related technology, applied in the direction of antiviral agents, applications, food science, etc., can solve the problems of inability to control the digestive system infection of the new coronavirus, low risk of clinical benefits, and affect work and life, etc., to achieve improvement Effects of intestinal barrier function, shortening the course of disease, and relieving symptoms of digestive system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082]Example 1: Application of lysozyme in patients with new coronal pneumonia and high risk infected population
[0083]During the Chinese New Crown Pneumonia epidemic, our company donated two more than 2 million yuan to Hubei Province, including lysozyme-containing tablets, lysozyme-coated tablets and other antibacterial drugs. These drugs play a positive role in fighting new champions. After many patients voluntarily tuned our company's lyse enzyme products.
[0084]9 new crown pneumonia newly diagnosed patients taking the lysozyme-containing sheet and / or lycipatinolic sheet, the dose of taking is 0.5g-2g daily, and the daily basically insists, and the results are only converted to In the case of a critical illness, the other 8 is mild, and then healed. The calculated concentration is 11.1%.
[0085]There are 3 people, during the epidemic, live and business trips in high-risk areas, insist on taking lysozyme-containing tablets and / or lycosolite tablets daily, and the amount is 1 g-1....
Embodiment 2
[0087]Example 2: In vitro test of lyse enzyme anti-new crown virus
[0088]The effect of lysease anti-COVID-19 new crown virus was studied with African green monkey renal cells.
[0089]Drugs: lysozyme, mono glycyrrhid acid.
[0090]Cells: African green monkey renal cells (VeroE6 cells).
[0091]Virus: COVID-19, saved by Guangzhou Customs Technology Center, using a titer of 100Tcid50.
[0092]Culture: Sterile 96-well culture plate, 100 μl of concentration of 100 μl of concentration of 2 × 10 per hole5Cells / ml VeroE6 cells were cultured at 37 ° C for 24 hours. The culture plate is divided into a blank control group, a viral control group, a low dose group, a lyse enzyme high dose group, a linsene acid mono metaphanylammonium, and a high-dose group of glycyrrhizic acid. 3 holes. In addition to the blank control group, 100Tcid is added in each group.50Virus 5μl / well, 5% CO at 37 ° C2After 2 hours of incubator, the cell culture medium in the culture plate was discarded.
[0093]Pharmaceutical: The dr...
Embodiment 3
[0098]Example 3: In vitro trial of the combined anti-neogous crown virus and new crown virus caused by
[0099]The inhibition of cytoplasms caused by lysozymes on cytoplasm caused by new crown viruses, as well as inhibitory effects of inflammation caused by new crown viruses.
[0100]Both drugs, cells, viruses, etc. are the same as in Example 2.
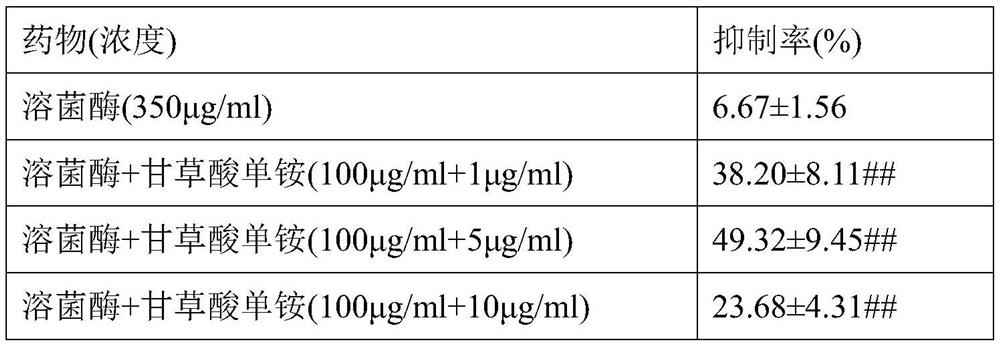
[0101]The culture plate group and the dose of the dosing were: blank control group, viral control group, lyse group (350 μg / ml), lycoshanol mono ammonium I group (100 μg / ml + 1 μg / mL), lyse enzyme glycyrrhizone Group II group (100 μg / ml + 5 μg / ml), a monogenic group (100 μg / ml + 10 μg / ml), lycozyme glycolic acid single ammonium IV group (100 μg / ml + 50 μg / mL).
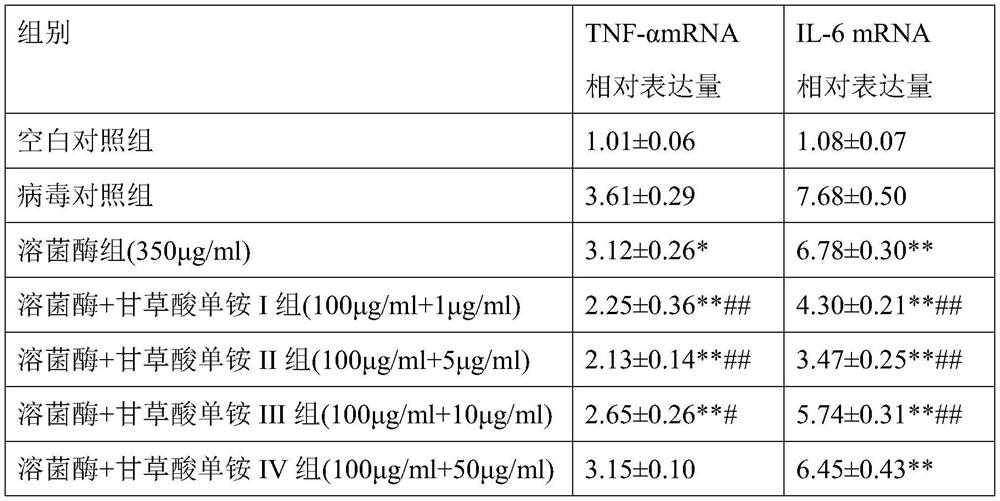
[0102]The observed cell lesions were the same as in Example 2, and the test results are shown in Table 2. In addition, non-pathogenic cells were collected, and RNA was extracted, and the relative expression levels of inflammatory factors such as TNF-α, IL-6 were determined by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com