Asarone nanocrystallization method
A kind of asarone and nanotechnology, which is applied in nanotechnology, nanotechnology, nanomedicine, etc., can solve the problems of low bioavailability and difficulty in dissolution of oral preparations of asarone, and achieve simple preparation process and high bioavailability in vivo Improvement, the effect of small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The nanometerization method of asarone
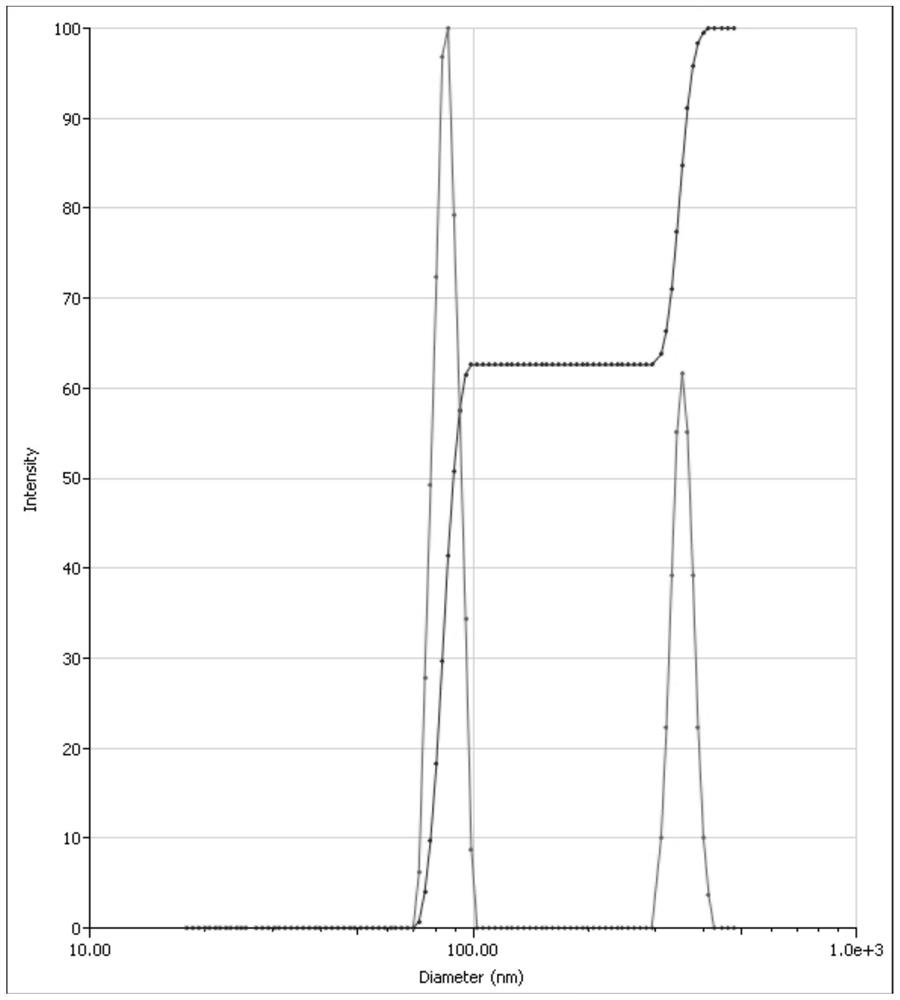
[0027] Weigh 0.250g of polyethylene glycol 15-hydroxystearate and 0.250g of polyvinyl alcohol, place them in a beaker, add about 50g of water to dissolve them completely, and prepare an aqueous stabilizer solution. Weigh 0.500g of asarone crude drug, place it in a 10mL sample bottle, add 5g of polyethylene glycol 400, shake to dissolve the drug, and make an organic phase. Under the conditions of probe ultrasound (2s on, 3s off), the parameters of ultrasound are power 60W, time 5min, absorb the organic phase with a dropper and add it into the stabilizer aqueous solution at a uniform speed to obtain the nano-scale colloidal dispersion of asarone. The particle size measured by the laser particle size analyzer is 119.37nm, and the PDI is 0.261, see figure 1 .
Embodiment 2
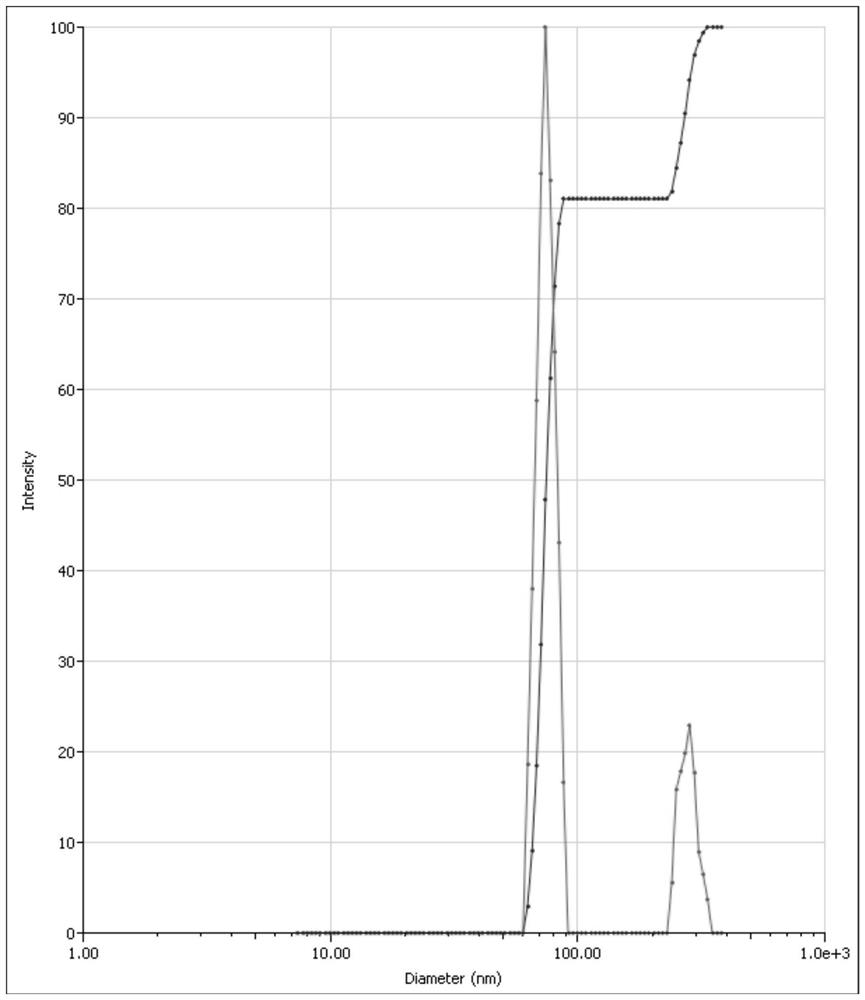
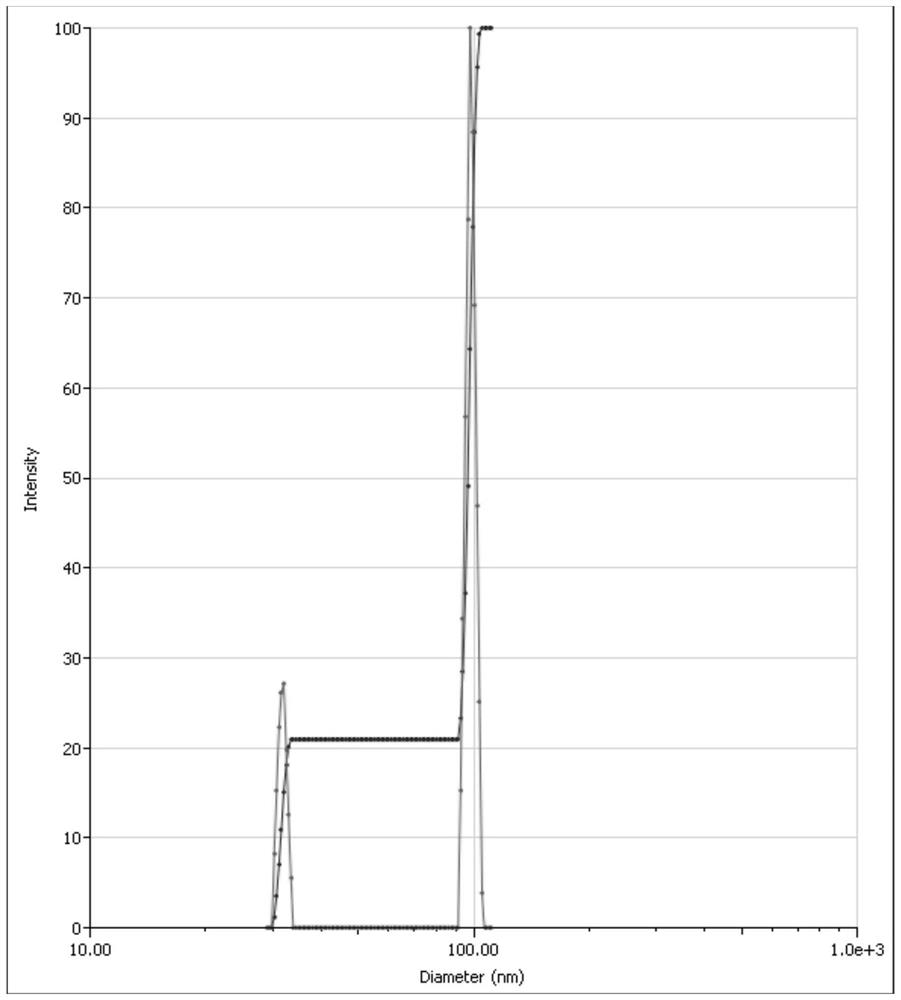
[0029] Effects of Probe Ultrasonic Power and Time on the Size of Asarone Nanoparticles
[0030] Weigh 0.050g of polyethylene glycol 15-hydroxystearate and 0.050g of polyvinyl alcohol, place them in a beaker, add about 50g of water to dissolve them completely, and prepare an aqueous stabilizer solution. Weigh 0.050g asarone crude drug, place in a 10mL sample bottle, add 5g polyethylene glycol 400, shake to dissolve the drug, and make an organic phase.
[0031] (1) Ultrasonic power
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com