Plate-type immunofluorescence kit for platelet antibody detection and preparation method thereof
An immunofluorescence and antibody detection technology, which is applied in biological testing, measuring devices, material inspection products, etc., can solve the problems of high laboratory requirements, and achieve the effects of high sensitivity, improved detection sensitivity, and strong result specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Selection of Characteristic Predominant Sequences of Coding Genes of HLA-Class I Mixed Antigens
[0033] The coding genes of HLA class I antigens were retrieved from IMGT / HLA.
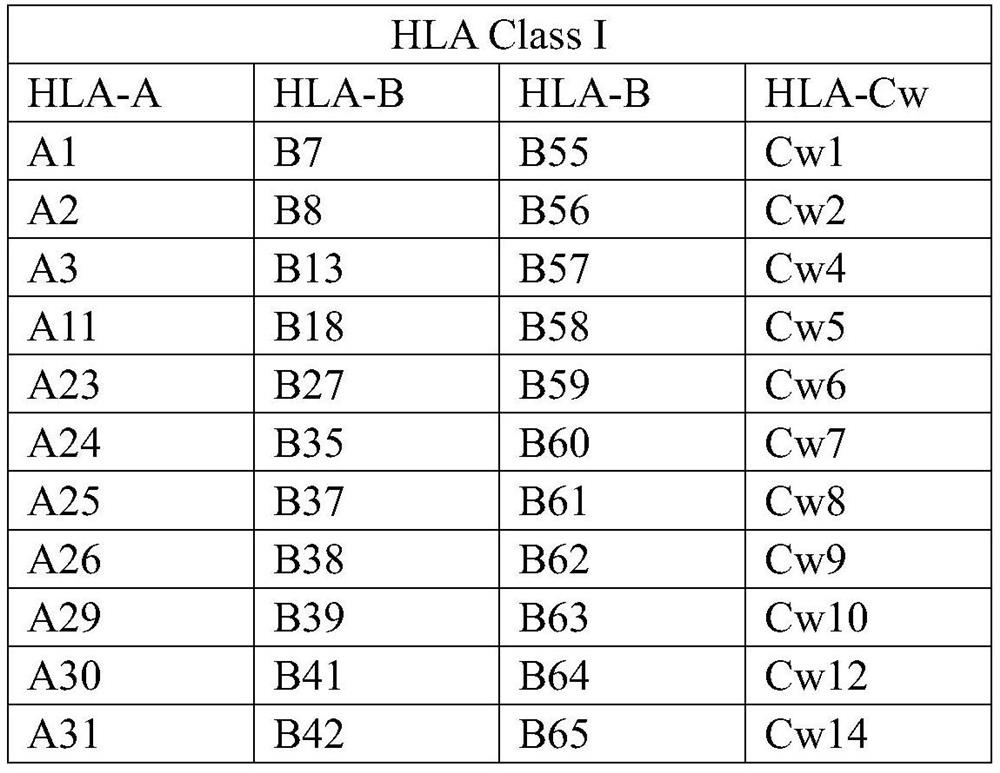
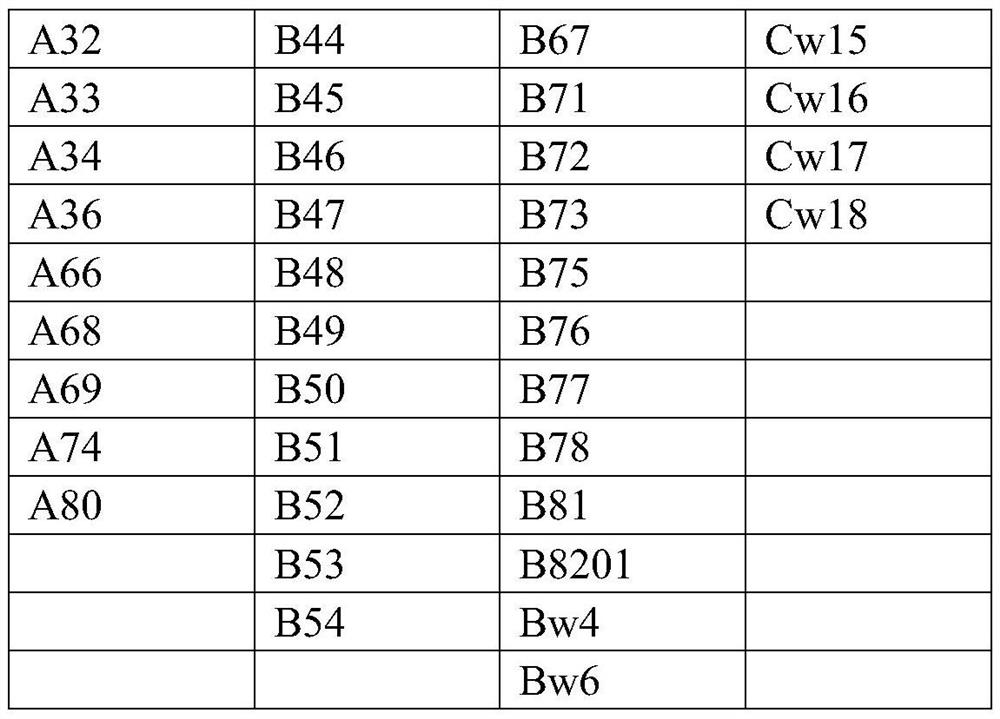
[0034] The antigenic determinant reactive epitopes of each antigenic protein were predicted by DNAstar-protean, and the gene fragments without reactive epitopes were excluded. Then use NetOGlyc and NetNGlyc to predict the N-glycosylation sites and O-glycosylation sites of the coding genes of each antigen protein, and exclude the reactive epitopes containing N-glycosylation sites and O-glycosylation sites fragments. Due to the high homology among the various antigens of the HLA-I class, the fragments with higher homology of the epitope of the antigenic determinant reaction epitope were selected as the HLA-I class mixed antigen in the coding genes of the remaining antigenic proteins. The characteristic dominant sequence is shown in Table 11:
[0035] Table 11 Characteristic sequences ...
Embodiment 2
[0039] The specific process of protein expression of the characteristic dominant sequence is as follows:
[0040] The designed characteristic dominant sequence was designed and synthesized by General Biosystems Co., Ltd. primers, and the restriction sites of BamH I and Xho I (Takara company) were added to the primer setting, and PCR amplification reaction was carried out to amplify Products were identified using 1.5% agarose gel electrophoresis. The purified PCR product and pcDNA3.1 were digested with BamH I and Xho I, and the separated target gene and plasmid pcDNA3.1 (Biobowell Biotechnology Co., Ltd.) were recovered by gel electrophoresis and treated with T4 DNA ligase (Takara Company) at 16°C. Overnight ligation reaction, take a small amount of linker to transform the competent cell DH5α and culture overnight, and the transformed bacteria are coated with LB solid medium with 100 μg / ml ampicillin (10g / L tryptone, 5g / L yeast extract, 10g / L chloride Sodium, pH=7.4) plates, i...
Embodiment 3
[0043] The protein samples after expression and purification of SEQ ID No.1-11 were diluted to 4 μg / mL and coated on the Elisa plate respectively, and each protein sample was coated with two reaction wells and control wells, 100 μL / well, 4 Coat at ℃ for 18h, wash twice with 200μL / well washing solution, block with 120μL / well blocking solution at 37℃ for 1h, pour off the blocking solution, and pat dry. Add 100 μL of HLA antibody-positive samples of the corresponding proteins in turn to the reaction wells (SEQ ID No.1: A1 positive sample, SEQ ID No.2: A25 positive sample, SEQ ID No.3: A68 positive sample, SEQ ID No.4: B7 Positive sample, SEQ ID No.5: B18 positive sample, SEQ ID No.6: C1 positive sample, SEQ ID No.7: C4 positive sample, SEQ ID No.8: C5 positive sample, SEQ ID No.9: C7 Positive sample, SEQ ID No.10: C17 positive sample, SEQ ID No.11: C18 positive sample, sample diluent diluted three times), add 100 μL sample diluent to the control well, room temperature (22-28°C), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com