Glimepiride dispersible tablet composition and preparation method thereof
A technology for glimepiride and dispersible tablets, which is applied in the field of medicine, can solve the problems of uneven dispersion, poor dissolution effect, influence on drug efficacy and the like, and achieves the effects of simple production process, good process tolerance and stable clinical efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
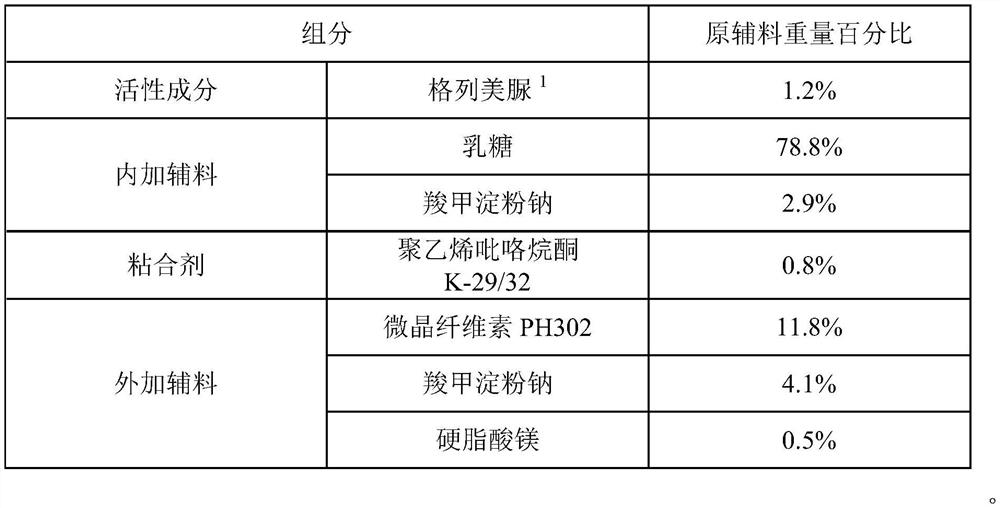
[0057] Embodiment 1: Glimepiride Dispersible Tablets
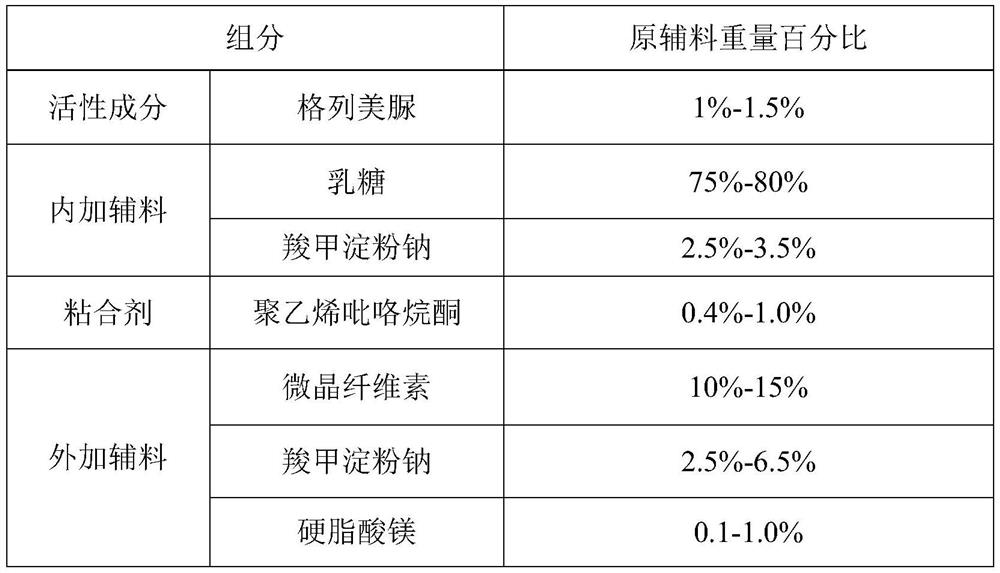
[0058] 1. Prescription composition:
[0059]
[0060] Feed intake according to the prescription quantity of 200000 tablets, prepare glimepiride dispersible tablet, the same below.
[0061] 2. Preparation method:
[0062] (1) Preparation of adhesive: polyvinylpyrrolidone K -29 / 32 Dissolved in water to prepare a 6.0% (w / w) binder solution;
[0063] (2) Premixing and wet granulation: add glimepiride and sodium carboxymethyl starch as an internal auxiliary material into the wet mixing granulator for premixing 1, add lactose into the wet mixing granulator for premixing 2. Then add the binder solution under stirring conditions, and wet granulate; the process parameters are as follows:
[0064]
[0065] (3) Drying: Dry the granules at 48-53°C until the water content is 1.5%;
[0066] (4) Grain sizing: add the dried granules to the sizing machine for sizing;
[0067] (5) Mixing: add microcrystalline cellulose PH302 and...
Embodiment 2
[0079] Embodiment 2: Glimepiride Dispersible Tablets
[0080] One, prescription composition: with embodiment 1
[0081] 2. Preparation method:
[0082] Preparation method: step (1)-step (5) is the same as embodiment 1;
[0083] (6) Tablet compression: the tablet compression pressure is 10KN; each tablet contains 1 mg of glimepiride in dispersible tablets.
[0084] 3. Test results:
[0085] 1. The friability, content uniformity and dispersion uniformity of the prepared glimepiride dispersible tablets were measured, and the results are shown in Table 4.
[0086] Table 2: Results of friability, content uniformity, and dispersion uniformity of glimepiride dispersible tablets in Example 2
[0087]
[0088] 2. The dissolution rate of the prepared glimepiride dispersible tablets was tested, and the results are shown in Table 5.
[0089] Table 5: Dissolution results of Glimepiride Dispersible Tablets in Example 2
[0090]
Embodiment 3
[0091] Embodiment 3: Glimepiride Dispersible Tablets
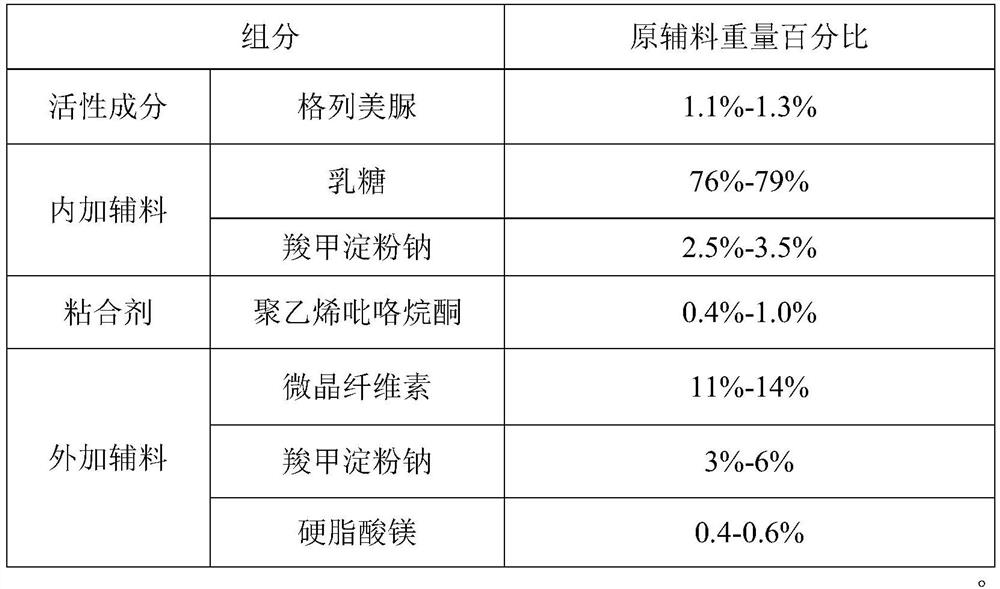
[0092] 1. Prescription composition:
[0093]
[0094] 2. Preparation method:
[0095] (1) Preparation of adhesive: polyvinylpyrrolidone K -29 / 32 Dissolved in water to prepare a 7.0% binder solution;
[0096] (2) Premixing and wet granulation: add glimepiride and sodium carboxymethyl starch as an internal auxiliary material into the wet mixing granulator for premixing 1, add lactose into the wet mixing granulator for premixing 2. Then add the binder solution under stirring conditions, and wet granulate; the process parameters are as follows:
[0097]
[0098] (3) Drying: Dry the granules at 45-50°C until the moisture content is 1.7%;
[0099] (4) Grain sizing: add the dried granules to the sizing machine for sizing;
[0100] (5) Mixing: add microcrystalline cellulose PH302 and the excipient sodium carboxymethyl starch to the granules, stir and mix for 8 minutes, then add magnesium stearate, and mix for 8 minutes;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com