Synthesis method of montelukast sodium intermediate
A technology of montelukast sodium and its synthesis method, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of cumbersome process steps, no treatment of three wastes, and low yield, and achieve the effects of simple process steps, high industrial value and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] In the embodiment of the present invention, the synthesis method of the intermediate of montelukast sodium includes:
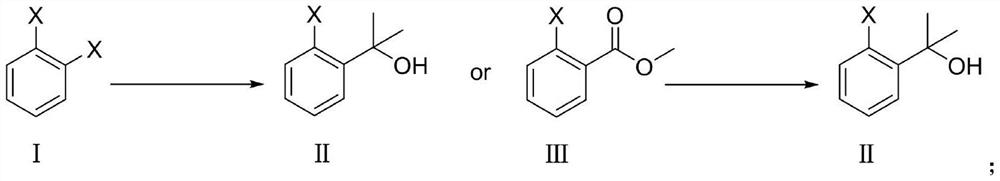
[0023] 1,2-dibromobenzene, 1,2-diiodobenzene, 1,2-dichlorobenzene or methyl 2-bromobenzoate, methyl 2-iodobenzoate, methyl 2-chlorobenzoate in tetrahydrofuran Under the action of , react at the temperature of -88°C ~ -68°C and -10°C ~ 10°C respectively to generate compound II, the reaction is as follows:
[0024]
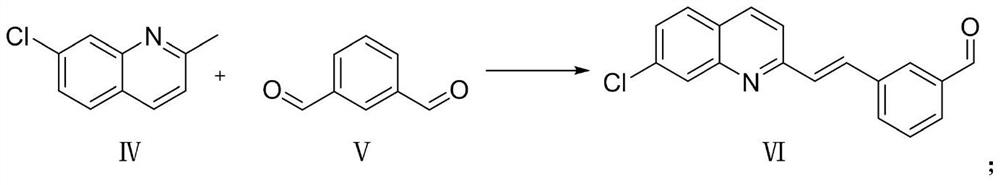
[0025] Compound IV and compound V are condensed under the action of acetic anhydride and triethylamine to obtain compound VI, wherein the reaction temperature is 100°C to 120°C, and toluene or xylene is used as a solvent for 8 to 12 hours. The reaction is as follows:
[0026]
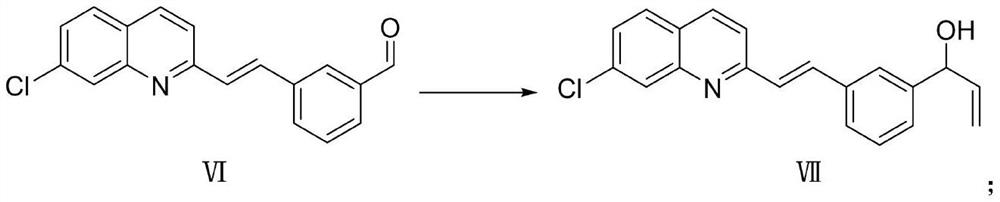
[0027] Compound VI is reacted with tetrahydrofuran as a solvent under the action of vinylmagnesium bromide at a temperature of -30°C to -10°C to generate compound VII, and the reaction is as follows:
[0028]
[0029] Compound VII and the compound II are cond...
Embodiment 1
[0065] Embodiment 1: Preparation of compound II (taking 1,2-dibromobenzene as an example)
[0066] Add 1,2-dibromobenzene (118g, 0.5mol) into anhydrous tetrahydrofuran (1L), stir and cool down to -78°C, add n-butyllithium (220mL, 2.5mol / L n-hexane as solvent) dropwise, continue After stirring for 1 h, anhydrous acetone (29 g) was added dropwise. After the dropwise addition was completed, the temperature was slowly raised to -30° C., and stirring was continued for 3 h. The reaction was quenched by adding water, and the organic phase was separated, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 100 g (yield 93%) of a nearly colorless liquid, which was compound II.
Embodiment 2
[0067] Embodiment 2: the preparation of compound II (taking 2-bromobenzoic acid methyl ester as example)
[0068] Methyl 2-bromobenzoate (226g, 1.05mol) was dissolved in THF (1.6L), and 3MCH was added dropwise in an ice-water bath 3 MgBr (1.05 L, 3.15 mol) was added dropwise to form an off-white slurry liquid, which was slowly raised to room temperature and stirred until the reaction was completed. Under ice-water bath conditions, use HCl (4.5L, 0.5 M, 2.25mol) to slowly quench the reaction, after the addition is complete, continue stirring for 0.5h, then add 2N HCl (0.5 L, 1.00mol) to make the pH value 5-6 . Add MTBE (1L), separate the organic phase, then extract the aqueous phase with MTBE (2×0.5L), combine the organic phases, wash with sodium bicarbonate solution (2×0.3L), dry over anhydrous sodium sulfate, filter, and concentrate , 222 g (98.2% yield) of compound II was obtained as a solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com