Compound preparation for external use for treating alopecia areata and preparation method thereof
An external preparation and compound technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as poor hair repair effect, reduce the risk of systemic side effects, and prolong the life of the drug. Effective, targeted effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
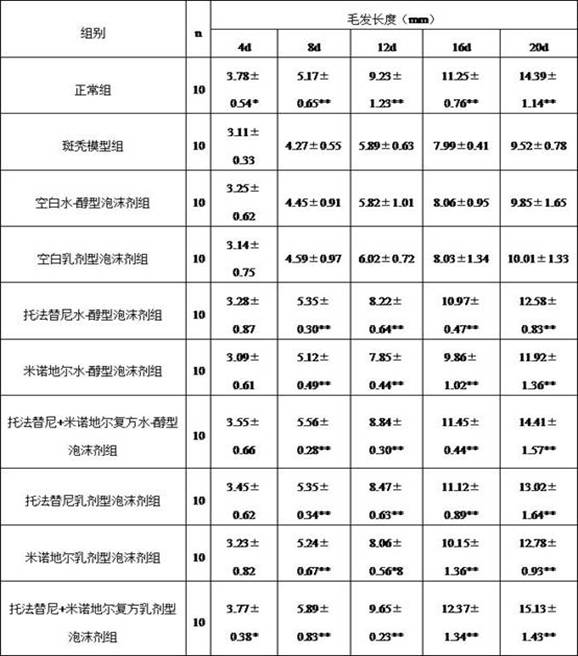
Examples
Embodiment 1
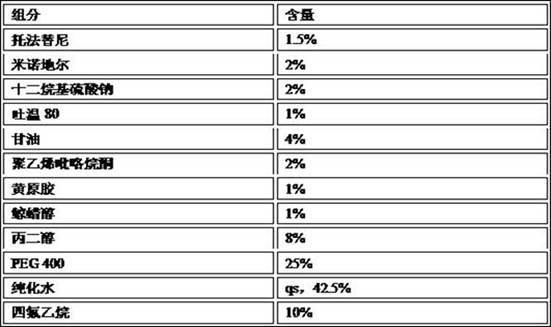
[0053] (1) Prescription
[0054]
[0055] (2) Preparation method
[0056] In the preparation container, add the prescribed amount of purified water, PEG 400 and propylene glycol, and stir evenly to form a mixed solvent. While stirring, slowly add Tween 80, sodium lauryl sulfate, tofacitinib, minoxidil, glycerin, polyvinylpyrrolidone, xanthan gum, cetyl alcohol in the prescribed amount, and then use a homogenizer to homogenize chemical or sonication. The prepared pre-foaming mixture is filled in a pressurized container tank and filled with tetrafluoroethane to obtain the product.
Embodiment 2
[0058] (1) Prescription
[0059]
[0060] (2) Preparation method
[0061] In the preparation container, add the prescribed amount of purified water, PEG 400 and ethanol, and stir evenly to form a mixed solvent. Slowly add the prescribed amount of Tween 80, sodium fatty alcohol polyoxyethylene ether sulfate, tofacitinib, minoxidil, glycerin, hypromellose, carbomer, stearyl alcohol while stirring, and then use Homogenize or ultrasonically dissolve. Put the prepared pre-foaming mixture into a hand-pressed net-type foam lotion pump bottle to get ready.
Embodiment 3
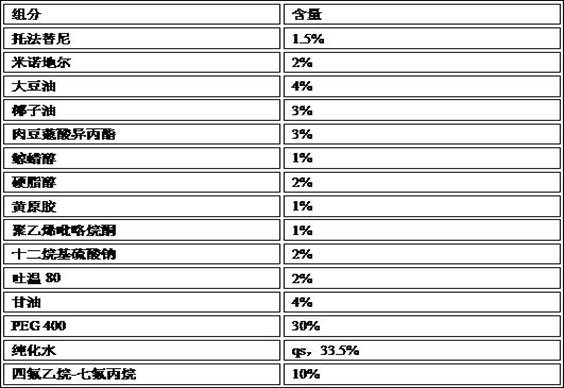
[0063] (1) Prescription
[0064]
[0065] (2) Preparation method
[0066] Preparation of aqueous phase mixture: Add the prescribed amount of xanthan gum, polyvinylpyrrolidone, sodium lauryl sulfate, Tween 80, glycerin, etc. into the mixed solvent of water and polyethylene glycol 400 while stirring, and heat to 50~ Dissolve at 70°C;
[0067] Preparation of oil phase mixture: Heat soybean oil, coconut oil, and isopropyl myristate to the same temperature as the water phase, and add prescribed amounts of cetyl alcohol, stearyl alcohol, tofacitinib, and minoxidil while stirring , to make it dissolve;
[0068] Mixing: Slowly pour the hot oil phase into the hot water phase under stirring, emulsify with a homogenizer, and cool to room temperature;
[0069] Filling: Fill the prepared pre-foaming mixture into a pressurized container tank and fill it with tetrafluoroethane-heptafluoropropane mixture to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com