Bionic exosome as well as preparation and application thereof
An exosome and bionic technology, applied in the field of bionic exosome, can solve problems such as undiscovered tumor treatment, achieve the effect of increasing tumor self-targeting ability, avoiding phagocytosis, and increasing circulation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] This example is used to illustrate the preparation of liposomes.
[0061] Liposomes were prepared by the thin film dispersion method. 20mg lipid material DPPC, cholesterol and DSPE-PEG 2000 (mass ratio 80:15:5) was dissolved in 2 mL of chloroform:methanol (3:1 V / V), and the solvent was evaporated by a rotary evaporator to form a lipid film.
[0062] Add 2mL PBS to the lipid film for hydration to form a lipid suspension.
[0063] The lipid suspension was passed through a cellulose acetate membrane with a pore size of 100 nm through a liposome extruder at 25°C for 20 times, and the obtained homogeneous liposome solution was stored at 4°C for use.
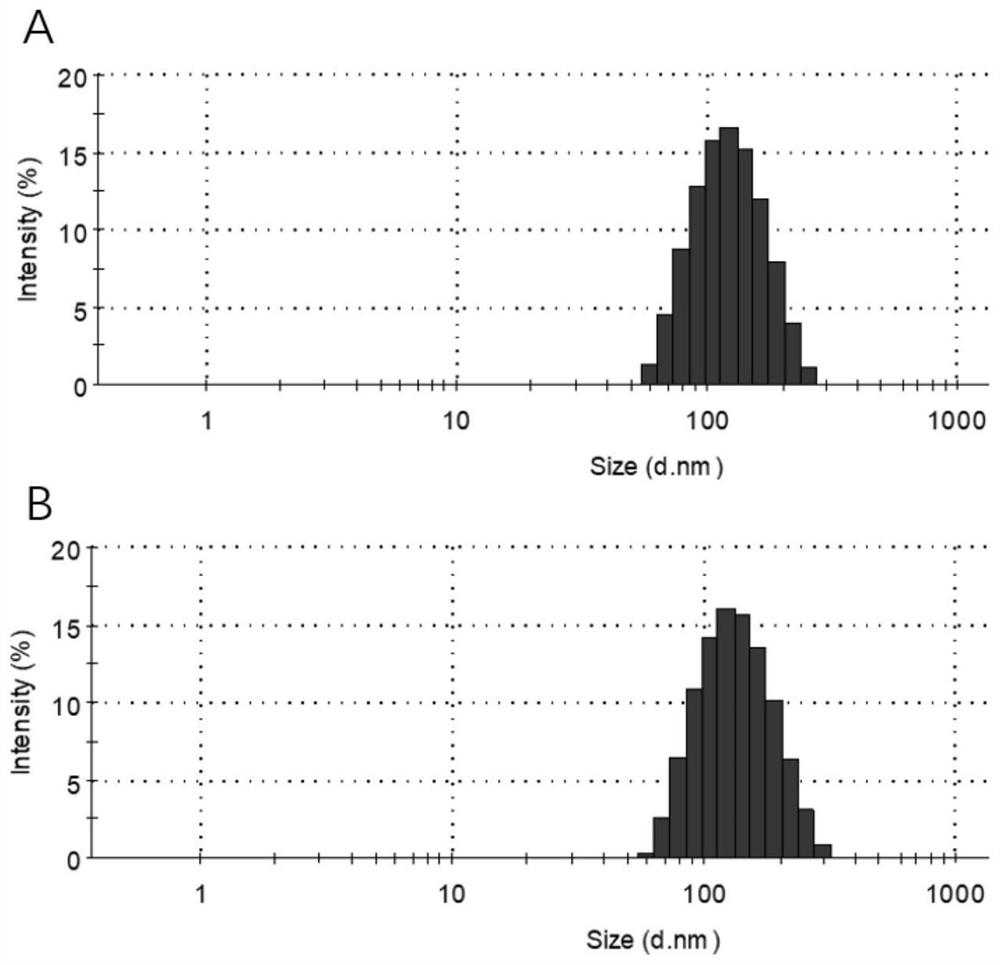
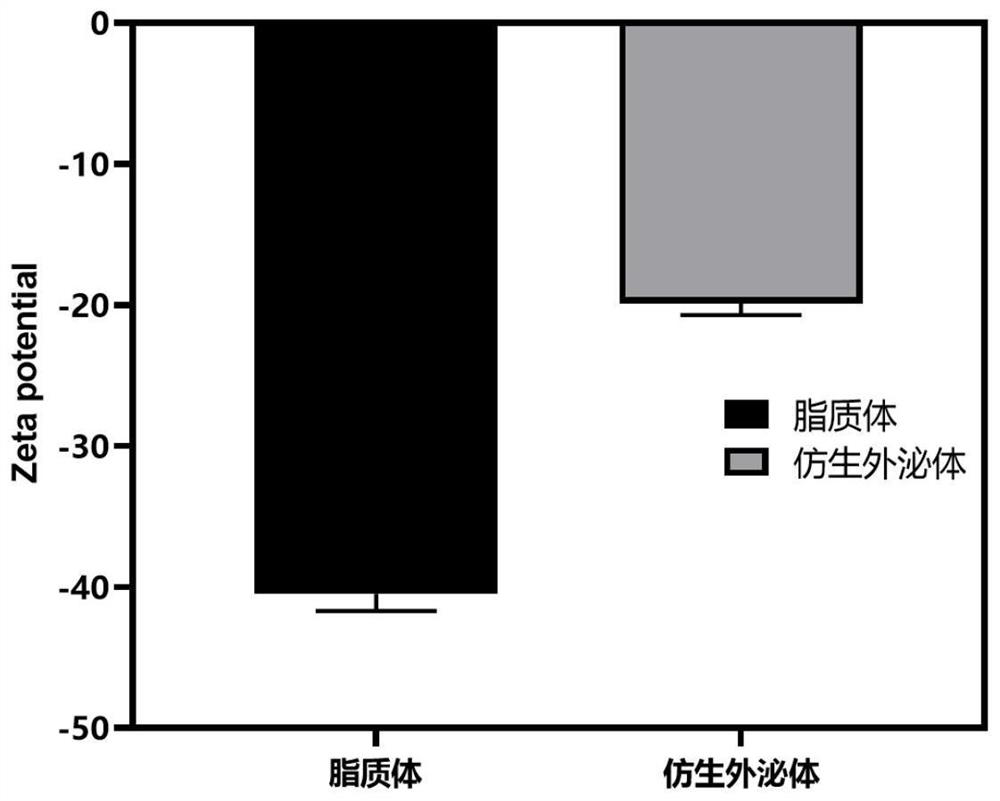
[0064] The particle size distribution of liposomes ( figure 1 A), zeta potential ( figure 2 ) was characterized by a nanometer particle size analyzer, and the morphology ( image 3 A) Characterized by transmission electron microscopy.
[0065] In the particle size distribution diagram, the average particle size of liposo...
Embodiment 2
[0067] This example is used to illustrate the preparation of biomimetic exosomes.
[0068] 4T1 breast tumor cell membrane protein was extracted by cell membrane protein and cytoplasmic protein extraction kit, and the concentration of cell membrane protein was determined by BCA method.
[0069] In Example 1, tumor cell membrane protein was added while PBS was added to the lipid film for hydration, the mass ratio of membrane protein to lipid material was 1:100, and the mixture was fully mixed to form a suspension.
[0070] Add NE to the membrane protein-lipid suspension, the mass ratio of NE to lipid material is 1:40, incubate at 25°C for 30min, then pass through a cellulose acetate membrane with a pore size of 100nm through a liposome extruder for 20 times , and the obtained homogeneous biomimetic exosome solution was stored at 4°C for future use.
[0071] Particle size distribution of biomimetic exosomes ( figure 1 B), Zeta potential ( figure 2 ) was characterized by a nan...
Embodiment 3
[0074] This example is used to illustrate the protein characterization of membrane protein chimeric liposomes in the biomimetic exosomes of the present invention.
[0075] The tumor cell membrane proteins on the biomimetic exosomes prepared in Example 2 of the present invention were determined by Coomassie brilliant blue staining. Configure 10% SDS polyacrylamide gel, load 10 μg of 4T1 breast tumor cell total protein, 4T1 breast tumor cell membrane protein, 4T1 breast tumor cell cytoplasmic protein and biomimetic exosomes, and infiltrate with Coomassie brilliant blue staining solution after electrophoresis Soak and stain for 2 hours, then decolorize with decolorizing solution. Take pictures to observe the protein bands between groups (results see Figure 4 ). The results showed that the protein of the biomimetic exosome was very similar to the membrane protein of 4T1 breast tumor cells, but significantly different from the cytoplasmic protein of 4T1 breast tumor cells, indic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com