Method for removing and recovering fluorine and chlorine from zinc sulfate solution
A technology of zinc sulfate solution, fluorine and chlorine, which is applied in the field of metallurgy and chemical industry, can solve the problems of high input cost, easy burning of plates, complicated process, etc., and achieve the effect of reducing desorption cost, increasing disposal cost, and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
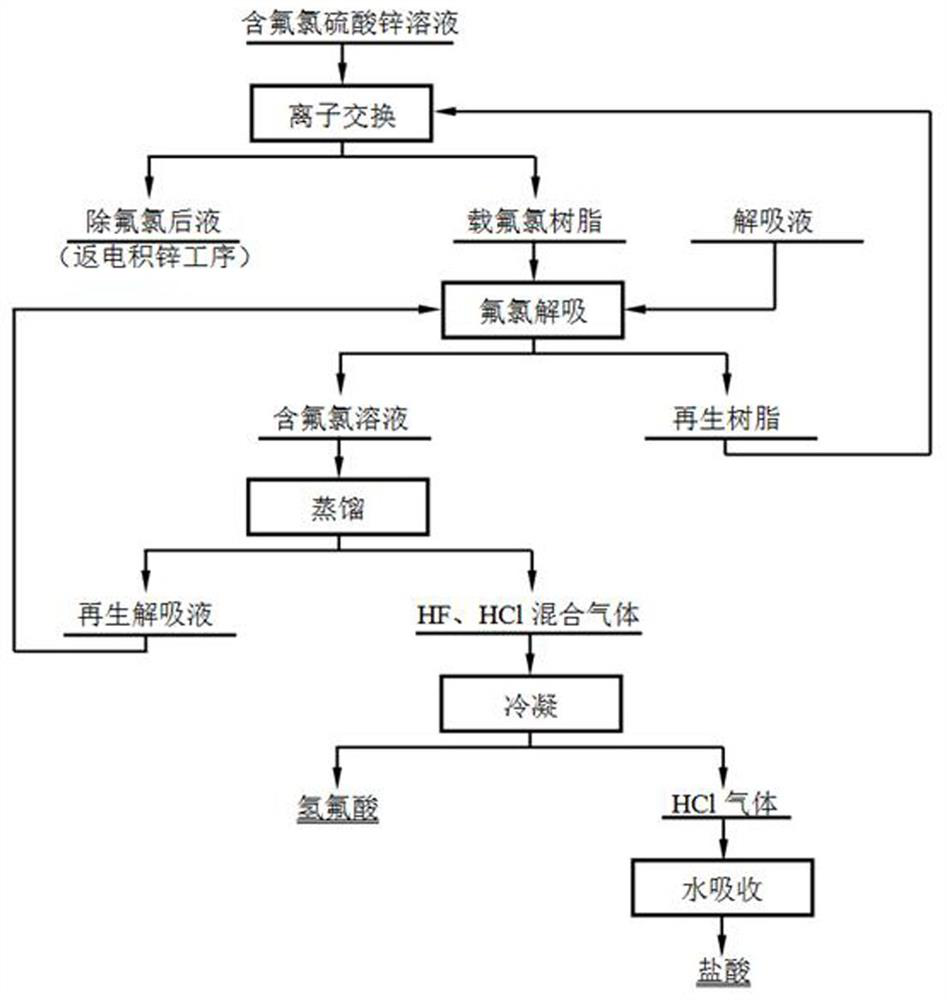
[0052] The main components of the fluorine-containing zinc chlorosulfate solution in this embodiment are Zn150g / L, Cl600mg / L, F200mg / L, and the pH of the solution is 2.5-3.0. 20 ion exchange columns (diameter 18mm, height 300mm), each resin column is filled with a mass ratio of 80%: 18%: 2% 201×7, D201, D301 resin mixture type anion resin, to control the flow of liquid It is 1L / h. A total of 20L of solution was processed. The main components of the solution after adsorption were Zn148.2g / L, Cl86.24mg / L, F52.5mg / L, the loss rate of zinc was 1.20%, the removal rate of chlorine was 85.63%, and the removal rate of fluorine was 68.75%.
[0053] The chlorine-fluorine-loaded resin is desorbed with 396g / L sulfuric acid solution, the flow rate of the feed liquid is controlled at 1L / h, and the amount of desorption liquid is 1.2L. After desorption, the liquid contains Cl4170mg / L, F1523mg / L, and the desorption rates of fluorine and chlorine are respectively 96.46% and 97.40%. After the ...
Embodiment 2
[0056] The main components of the fluorine-containing zinc chlorosulfate solution in this embodiment are Zn113g / L, Cl440mg / L, F150mg / L, and the pH of the solution is 2.5-3.0. 5 ion exchange columns (diameter 2300mm, height 5400m), each resin column is filled with a mass ratio of 80%: 15%: 5% 201×7, D201, D301 resin mixture type anion resin. Control the liquid flow rate of 10m 3 / h, the solution temperature is 60~70°C, and the total treatment solution is 240m 3 The main components of the mixed liquid after adsorption are Zn110g / L, Cl21mg / L, F13mg / L, the loss rate of zinc is 2.65%, the removal rate of chlorine is 95.45%, and the removal rate of fluorine is 93.75%.
[0057] The fluorine- and chlorine-loaded resin is desorbed with 196g / L (2mol / L) sulfuric acid solution, and the influent flow rate is controlled at 5 m 3 / h, the amount of desorption liquid is 30L, the desorption liquid contains Cl3840mg / L, F1195mg / L, and the desorption rates of fluorine and chlorine are 96.98% and...
Embodiment 3
[0060] The main components of the fluorine-containing zinc chlorosulfate solution in this embodiment are Zn180g / L, Cl1000mg / L, F800mg / L, and the solution pH2.5~3.0. 20 ion exchange columns (diameter 18mm, height 300mm), each resin column is filled with a mass ratio of 87%: 10%: 3% 201×7, D201, D301 resin mixture type anion resin, to control the inflow flow It is 1L / h. A total of 20L of solution was processed. The main components of the solution after adsorption were Zn148.2g / L, Cl86.24mg / L, F52.5mg / L, the loss rate of zinc was 1.20%, the removal rate of chlorine was 85.63%, and the removal rate of fluorine was 68.75%.
[0061] Fluorine- and chlorine-loaded resin is desorbed with 396g / L sulfuric acid solution, the feed flow rate is controlled to be 1L / h, and the desorption liquid consumption is 1.2L. After desorption, the liquid contains Cl4480mg / L, F2015mg / L, and the desorption rates of fluorine and chlorine are respectively 97.68% and 98.12%. After desorption and regenerati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com