Method for electrocatalytically and selectively reducing alkyne impurities in olefin
A selective and electrocatalytic technology, applied in the direction of electrodes, electrolysis process, electrolysis components, etc., can solve the problems of poor alkyne conversion rate and selectivity, no commercial feasibility, low solubility of olefins, etc., to achieve low cost, Excellent product selectivity, the effect of improving the conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
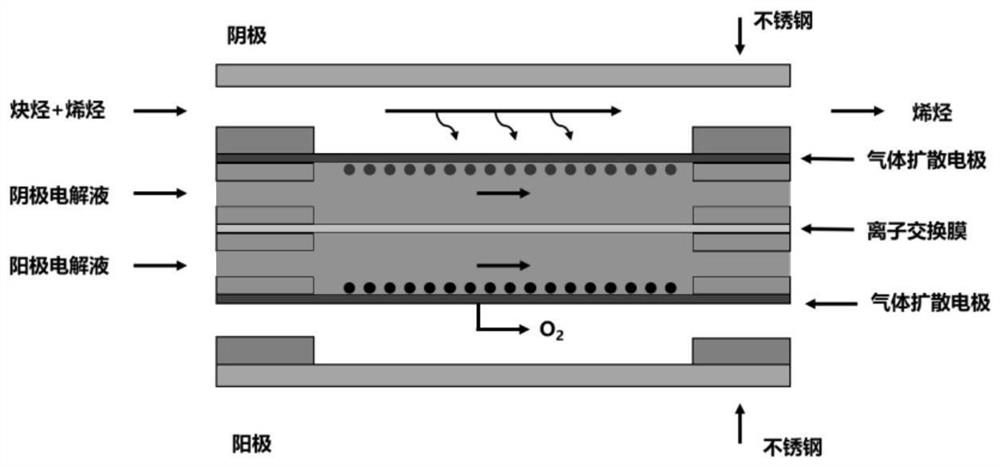
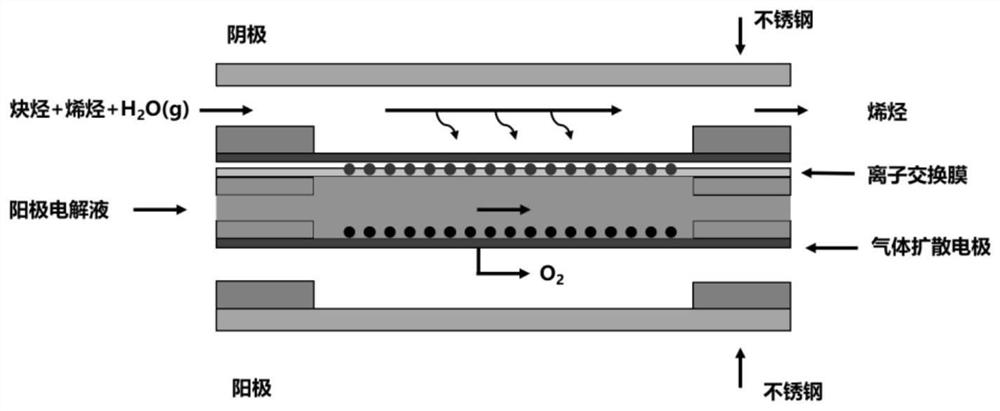
[0057] (1) The gas diffusion electrode made of Fe nanoparticles is used as the cathode of the electrolytic cell; the gas diffusion electrode made of iridium oxide catalyst is used as the anode of the electrolytic cell; both the catholyte and the anolyte are 1M KOH solution, and an anion is used between exchange membrane isolation, and as figure 1 The schematic diagram of the apparatus shown assembles the various components.
[0058] (2) Use a gas mass flowmeter to control the flow rate of the acetylene-ethylene mixed reaction gas to 50 sccm.
[0059] (3) A peristaltic pump is used to control the flow rate of catholyte and anolyte to 50 sccm.
[0060] (4) The catalytic activity of Fe nanoparticles was characterized by potentiostatic method.
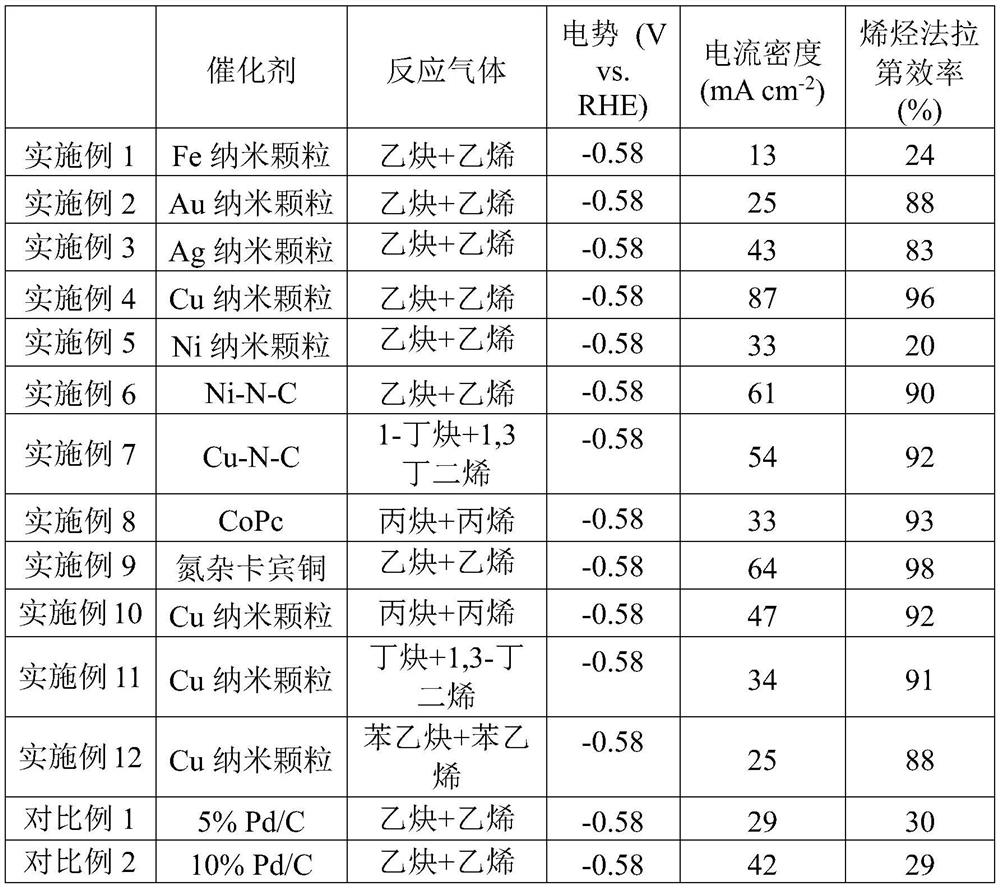
[0061] The catalyst composition and specific evaluation results are shown in Table 1.
Embodiment 2
[0063] (1) The gas diffusion electrode made of Au nanoparticles is used as the cathode of the electrolytic cell; the gas diffusion electrode made of iridium oxide catalyst is used as the anode of the electrolytic cell; both the catholyte and the anolyte are 0.5M H 2 SO 4 solution, separated by a proton exchange membrane, and as figure 1 The schematic diagram of the apparatus shown assembles the various components.
[0064] (2) Use a gas mass flowmeter to control the flow rate of the acetylene-ethylene mixed reaction gas to 50 sccm.
[0065] (3) A peristaltic pump is used to control the flow rate of catholyte and anolyte to 50 sccm.
[0066] (4) The catalytic activity of Au nanoparticles was characterized by potentiostatic method.
[0067] The catalyst composition and specific evaluation results are shown in Table 1.
Embodiment 3
[0069] (1) The gas diffusion electrode made of Ag nanoparticles is used as the cathode of the electrolytic cell; the gas diffusion electrode made of iridium oxide catalyst is used as the anode of the electrolytic cell; both the catholyte and the anolyte are 1M KOH solution, and an anion is used between exchange membrane isolation, and as figure 1 The schematic diagram of the apparatus shown assembles the various components.
[0070] (2) Use a gas mass flowmeter to control the flow rate of the acetylene-ethylene mixed reaction gas to 50 sccm.
[0071] (3) A peristaltic pump is used to control the flow rate of catholyte and anolyte to 50 sccm.
[0072] (4) The catalytic activity of Ag nanoparticles was characterized by potentiostatic method.
[0073] The catalyst composition and specific evaluation results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com