Enzalutamide soft capsule quick-release preparation and preparation method thereof
A technology of enzalutamide and immediate-release preparations, which is applied in the field of medicine, can solve the problems of increasing the instability of raw materials, difficulty of filling, and low drug loading, and achieve increased drug dispersion, high bioavailability, and improved bioavailability. The effect of utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
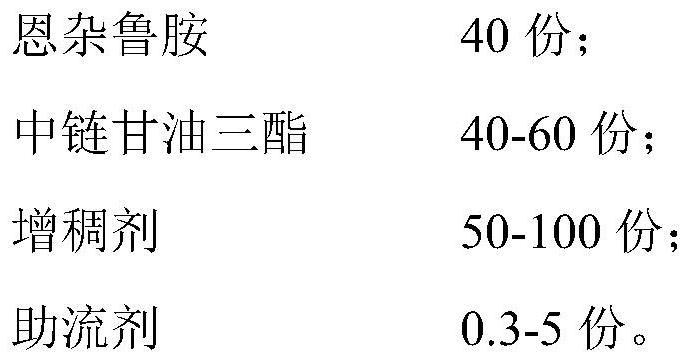
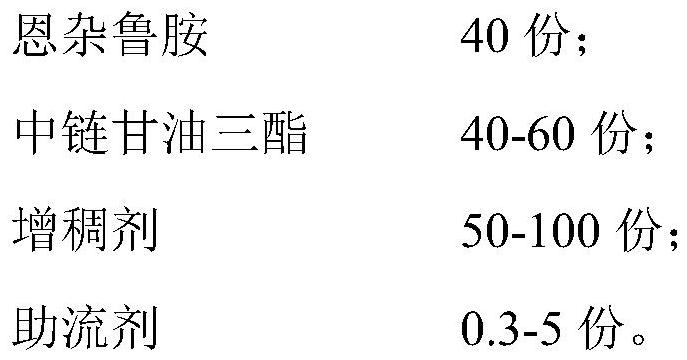
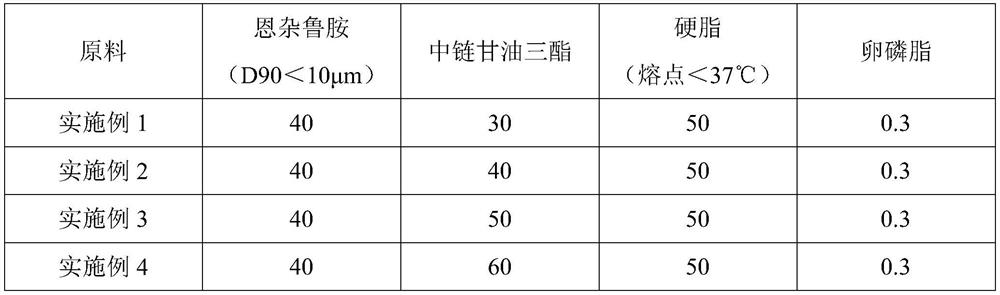
[0024] In Examples 1-10, the composition of the content and raw materials of the enzalutamide soft capsule immediate-release preparation is shown in Table 1.
[0025] The content raw material composition of table 1 embodiment 1-10 (in parts by weight)
[0026]
[0027]
[0028] The preparation method is as follows:
[0029] (1) heating the medium-chain triglyceride to 60° C., adding stearin and lecithin, and stirring evenly to obtain a pseudoplastic matrix fluid;
[0030] (2) Add enzalutamide to the pseudoplastic matrix fluid, stir evenly, and obtain a content suitable for soft capsule filling with certain fluidity;
[0031] (3) According to the mass ratio of gelatin, glycerin and purified water as 1:0.4:1, first add purified water and glycerin to the glue tank and heat to 70°C, stir until the solution is clear; then add gelatin to it, 70°C Stir to dissolve, and evacuate the air bubbles to obtain the capsule shell material;
[0032] (4) The contents and the capsule sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com