Fluorescent ceramic for warm white light illumination and preparation method thereof
A fluorescent ceramic and warm white light technology, which is applied in the chemical industry, can solve the problems of high equipment and environmental requirements, complicated preparation conditions, and reduced light extraction efficiency, and achieve good consistency, high controllability, and uniform ion distribution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Terbium oxide (chemical formula: Tb 4 o 7 ), alumina (chemical formula: Al 2 o 3 ), silicon dioxide (chemical formula: SiO 2 ), cerium oxide (chemical formula: CeO 2 ) and manganese dioxide (chemical formula: MnO 2 ) as raw material, take terbium oxide 209272g, aluminum oxide 9.5038g, silicon dioxide 0.0112g, cerium dioxide 0.0019g, manganese dioxide 0.0162g. The raw materials were mixed by ball milling in 18ml of absolute alcohol, wherein 90g of agate balls were added to the ball mill jar to assist in mixing evenly. The mixed slurry was ball milled at a speed of 250 r / min for 12 hours at room temperature.
[0040] After the ball milling was stopped, the slurry was dried in a drying oven at 80° C. for 12 hours to obtain a dried powder, and the dried powder was sieved with a 200-mesh nylon mesh to obtain a fine powder. Put the fine powder into a crucible and burn it in a muffle furnace at 850°C for 5 hours.
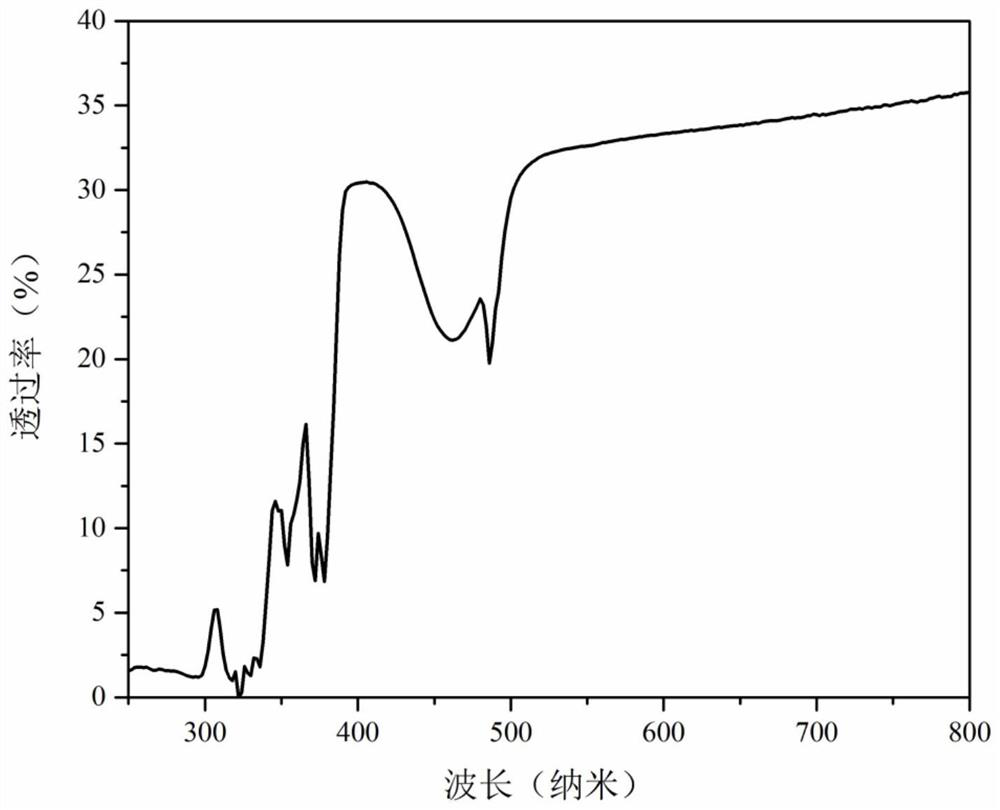
[0041] Put the bisque-fired powder into a metal film w...
Embodiment 2
[0049] The method for preparing fluorescent ceramic material in embodiment 2 is basically the same as that of example 1, and the raw materials taken are all terbium oxide, aluminum oxide, silicon dioxide, cerium dioxide and manganese dioxide, and the difference is that Mn 2+ , Si 4+ Relative to Al 3+ The doping amount is 0.015.
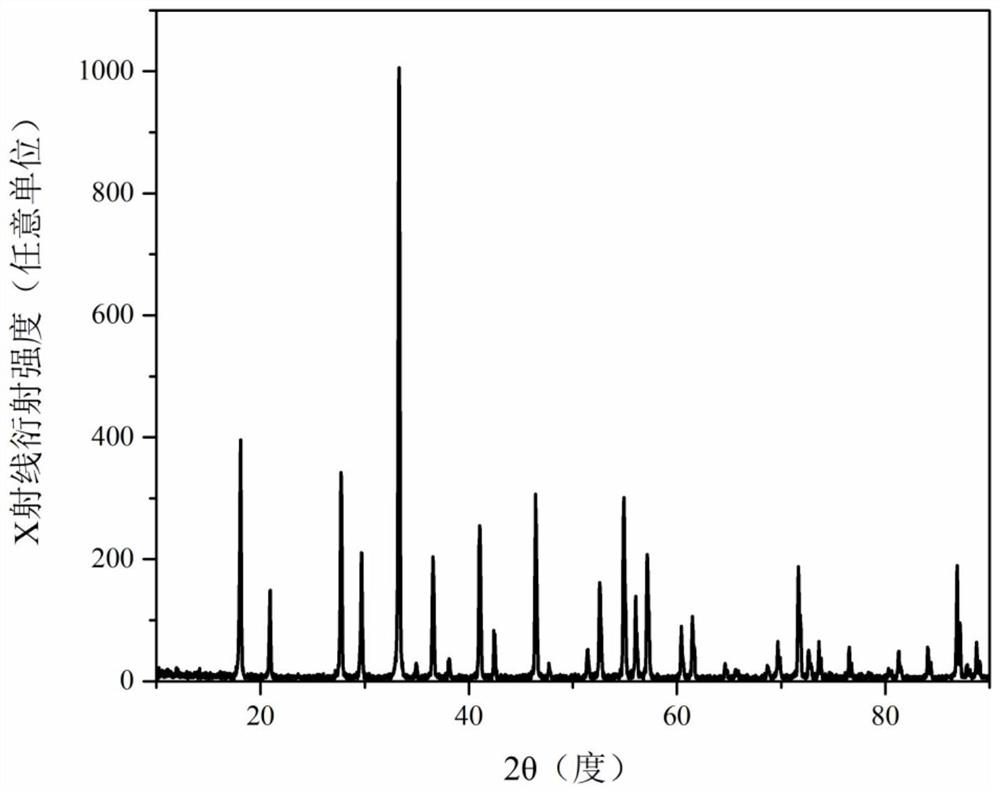
[0050] The phase composition of the obtained fluorescent ceramics is detected by X-ray diffractometer, and the phase of the obtained fluorescent ceramics is still a cubic garnet phase according to the test result data, and the XRD spectrum is as follows Image 6 shown.
[0051] Using a fluorescence spectrometer, test the fluorescence spectrum of the fluorescent material prepared in this embodiment under the excitation of 460nm blue light, the test results are as follows Figure 7 shown. A broadband light emission with a peak value of 585nm and a full width at half maximum of 134nm was obtained.
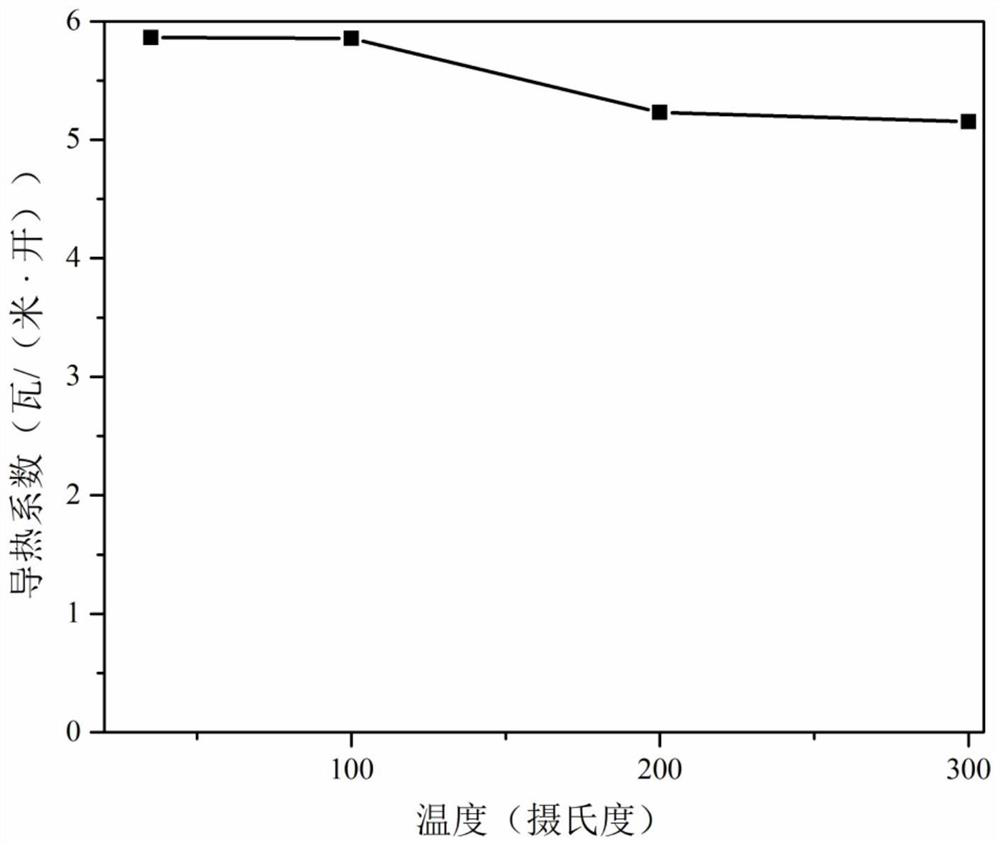
[0052] Using integrating sphere equipment, the ele...
Embodiment 3
[0054] The method for preparing fluorescent ceramic material in embodiment 3 is basically the same as that of example 1, and the raw materials taken are all terbium oxide, aluminum oxide, silicon dioxide, cerium dioxide and manganese dioxide, and the difference is that Mn 2+ , Si 4+ Relative to Al 3+ The doping amount is 0.025.
[0055] The phase composition of the obtained fluorescent ceramics is detected by X-ray diffractometer, and the phase of the obtained fluorescent ceramics is still a cubic garnet phase according to the test result data, and the XRD spectrum is as follows Figure 9 shown.
[0056] Using a fluorescence spectrometer, test the fluorescence spectrum of the fluorescent material prepared in this embodiment under the excitation of 460nm blue light, the test results are as follows Figure 10 shown. A broadband light emission with a peak at 590nm and a full width at half maximum of 132nm was obtained.
[0057] Using integrating sphere equipment, the electro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com