Method for detecting disodium ethylene diamine tetraacetate contained in acetylcysteine aerosol inhalation solution
A technology of disodium ethylenediaminetetraacetate and acetylcysteine, which is applied in measurement devices, instruments, scientific instruments, etc., can solve the problem of inability to establish a test method for the test sample, affecting the generation of reaction products, and interfering with the detection of target products, etc. problems, to achieve the effect of ensuring product quality, stable response, and quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
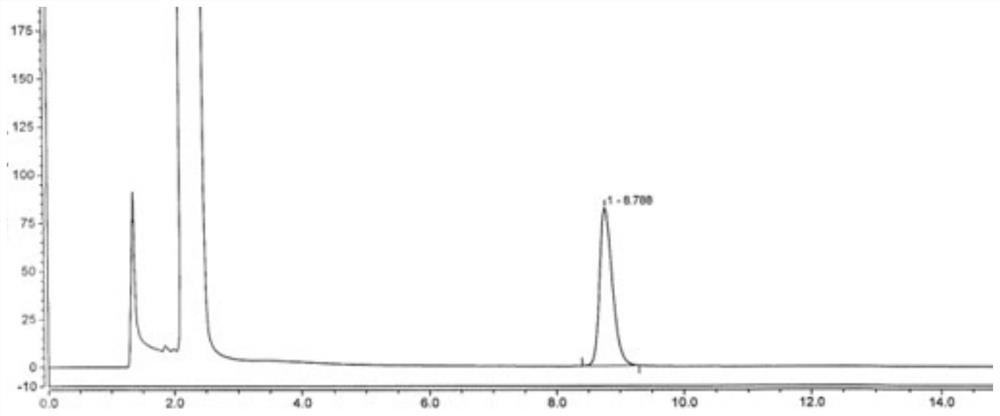
[0038] Embodiment 1: detection method of the present invention
[0039] Chromatographic column: Octadecylsilane bonded silica gel (Waters ODS-2 column, 4.0mm×250mm, 5μm);
[0040] Mobile phase A: Accurately measure 5.0 mL of 40% tetrabutylammonium hydroxide solution, add water to dilute to 1000 mL, and adjust the pH value to 2.5 with phosphoric acid;
[0041] Mobile phase B: acetonitrile;
[0042] Column temperature: 30°C;
[0043] Detector: UV detector;
[0044] Flow rate: 1.0mL / min;
[0045] Detection wavelength: 260nm;
[0046] Injection volume: 20μL;
[0047] Elution method: isocratic elution, the volume ratio of mobile phase A and mobile phase B is 88:12.
[0048] Preparation of reference solution: newly prepared before use. Take an appropriate amount of disodium edetate reference substance, add water to dissolve and quantitatively dilute to make a solution containing about 1.0 mg per 1 mL, and use it as a disodium edetate stock solution. Weigh about 40 mg of vita...
Embodiment 2
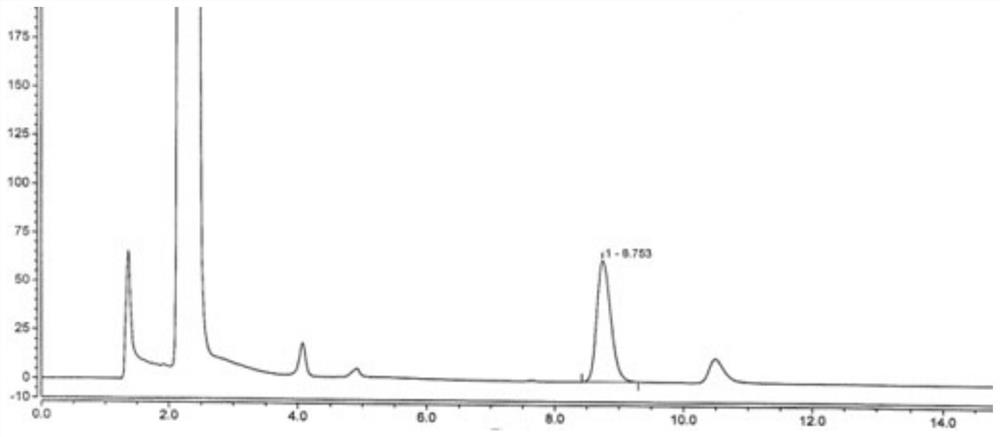
[0053] Embodiment 2: Investigation of the specificity of the detection method of the present invention
[0054] Preparation of reference solution: newly prepared before use. Take an appropriate amount of disodium edetate reference substance, add water to dissolve and quantitatively dilute to make a solution containing about 1.0 mg per 1 mL, and use it as a disodium edetate stock solution. Weigh about 40 mg of vitamin C, put it in a 25 mL measuring bottle, accurately add 1.0 mL of disodium edetate stock solution, add 15 mL of water to dissolve, shake well, add 2.0 mL of 0.1mol / L ferric chloride solution, shake well, Add water to dilute to the mark, shake well, that is.
[0055] Preparation of the test solution: freshly prepared before use. Weigh about 40 mg of vitamin C, put it in a 25 mL measuring bottle, accurately add 1.0 mL of acetylcysteine atomized inhalation solution, add 15 mL of water to dissolve, shake well, add 2.0 mL of 0.1mol / L ferric chloride solution, shake wel...
Embodiment 3
[0059] Embodiment 3: Inspection of the linear range of the detection method of the present invention
[0060] Take an appropriate amount of disodium edetate reference substance, add water to dissolve and quantitatively dilute to make a solution containing about 1.0 mg per 1 mL, and use it as a disodium edetate stock solution. Weigh 5 parts of vitamin C, about 40 mg each, and put them into five 25 mL measuring bottles, add 0.5 mL, 0.8 mL, 1.0 mL, 1.5 mL, 2.0 mL of disodium edetate stock solution precisely, and then Add 15mL of water to dissolve, shake well, add 2.0mL of 0.1mol / L ferric chloride solution, shake well, add water to dilute to the mark, shake well, as a series of linear solutions. Precisely measure the above-mentioned series of linear solutions, according to the chromatographic conditions of Example 1, sequentially inject and analyze from low concentration to high concentration, record the chromatogram, take the concentration C (μg / mL) of disodium edetate reference ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com