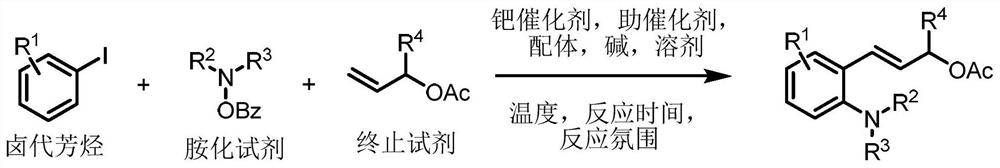
Preparation method of 2-amino cinnamyl alcohol ester derivative
A technology of cinnamyl alcohol ester and derivatives is applied in the field of preparation of 2-aminocinnamyl alcohol ester derivatives, and achieves the effects of simple operation steps, convenient post-processing and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of (E)-3-(2-methyl-6-morpholinophenyl)allyl acetate
[0031]
[0032] Palladium chloride 0.01mmol, tris(2-furyl)phosphine 0.025mmol, norbornene 0.2mmol, cesium carbonate 0.25mmol, o-methyl iodobenzene 0.1mmol, morpholinobenzoic acid 0.18mmol, allyl acetate 0.2 mmol, toluene 1mL was added to a 15mL reaction tube, nitrogen gas was repeatedly filled 10 times, placed in an oil bath at 80°C, and reacted for 24h; cooled to room temperature, filtered, concentrated, and purified by thin layer chromatography to obtain 24.4mg of the target product, the yield was 89%. The NMR characterization of this compound is as follows: 1 H NMR (500MHz, CDCl3) δ7.14(t, J=7.8Hz, 1H), 6.92(d, J=7.5Hz, 1H), 6.87(d, J=8.0Hz, 1H), 6.80(d, J =16.4Hz, 1H), 6.12(dt, J=16.4, 6.4Hz, 1H), 4.76(dd, J=6.4, 1.1Hz, 2H), 3.82-3.78(m, 4H), 2.95-2.91(m, 4H), 2.36(s, 3H), 2.10(s, 3H); 13 C NMR (126 MHz, CDCl3) δ 170.7, 151.3, 137.0, 130.9, 130.1, 127.7, 127.5, 125.5, 115.9, 67.2, 65.7, 52.3, 2...
Embodiment 2
[0034] Preparation of (E)-3-(2-ethyl-6-morpholinophenyl)allylacetate
[0035]
[0036] Palladium chloride 0.01mmol, tris(2-furyl)phosphine 0.025mmol, norbornene 0.2mmol, cesium carbonate 0.25mmol, o-ethyl iodobenzene 0.1mmol, morpholinobenzoic acid 0.18mmol, allyl acetate 0.2 mmol, toluene 1mL was added to a 15mL reaction tube, nitrogen gas was repeatedly filled 10 times, placed in an oil bath at 80°C, and reacted for 24h; cooled to room temperature, filtered, concentrated, and purified by thin layer chromatography to obtain 21.9mg of the target product, yield was 76%. The NMR characterization of this compound is as follows: 1 H NMR (500MHz, CDCl 3 )δ7.19(t, J=7.8Hz, 1H), 6.96(d, J=7.5Hz, 1H), 6.87(d, J=7.9Hz, 1H), 6.78(d, J=16.4Hz, 1H) , 6.14(dt, J=16.3, 6.4Hz, 1H), 4.75(dd, J=6.4, 1.2Hz, 2H), 3.92-3.61(m, 4H), 3.09-2.88(m, 4H), 2.71(q , J=7.5Hz, 2H), 2.11(s, 3H), 1.19(t, J=7.5Hz, 3H); 13 C NMR (126MHz, CDCl 3 )δ 170.8, 151.3, 143.3, 130.7, 129.8, 128.0, 127.2, 123.9...
Embodiment 3
[0038] Preparation of (E)-3-(2-isopropyl-6-morpholinophenyl)allyl acetate
[0039]
[0040] Palladium chloride 0.01mmol, tris (2-furyl) phosphine 0.025mmol, norbornene 0.2mmol, cesium carbonate 0.25mmol, 2-iodocumene 0.1mmol, morpholino benzoic acid 0.18mmol, allyl acetate Add 0.2mmol of ester and 1mL of toluene into a 15mL reaction tube, fill it with nitrogen gas repeatedly 10 times, place it in an oil bath at 80°C, and react for 24h; cool to room temperature, filter, concentrate, and purify by thin-layer chromatography to obtain 21.4mg of the target product. The yield was 71%. The NMR characterization of this compound is as follows: 1 H NMR (500MHz, CDCl 3 )δ7.24(t, J=7.9Hz, 1H), 7.06(d, J=7.8Hz, 1H), 6.87(d, J=7.9Hz, 1H), 6.77(d, J=16.3Hz, 1H) , 6.00(dt, J=16.3, 6.4Hz, 1H), 4.76(d, J=6.4Hz, 2H), 4.00-3.66(m, 4H), 3.44-3.19(m, 1H), 2.97-2.89(m , 4H), 2.11(s, 3H), 1.20(d, J=6.9Hz, 6H); 13 C NMR (126MHz, CDCl 3 )δ 170.9, 151.1, 147.9, 130.7, 129.9, 128.2, 127.6, 120.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com