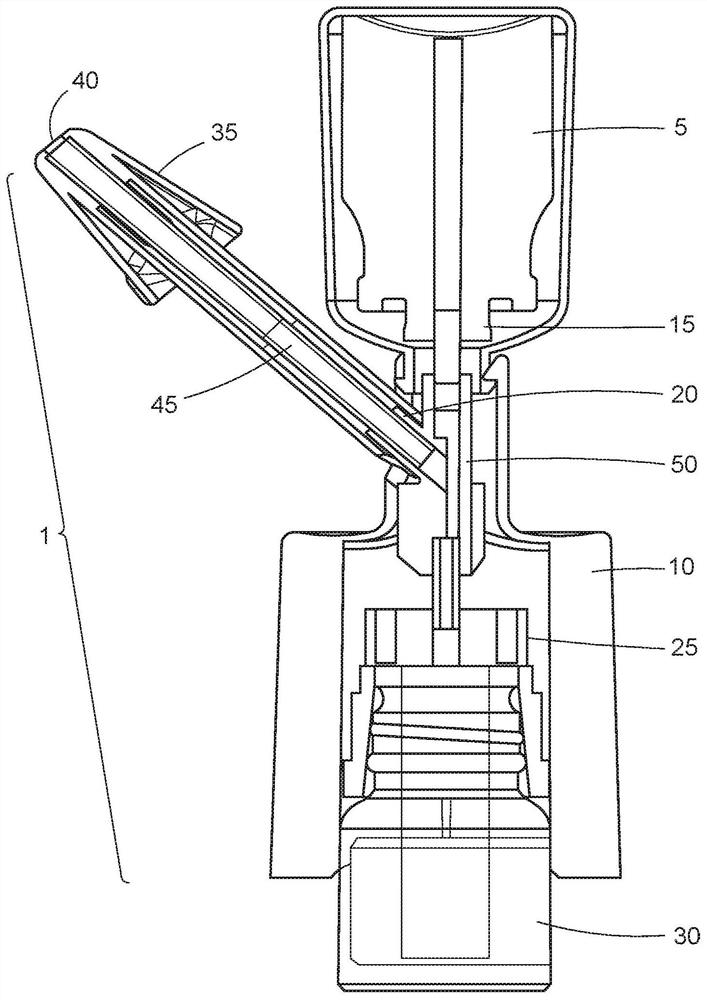
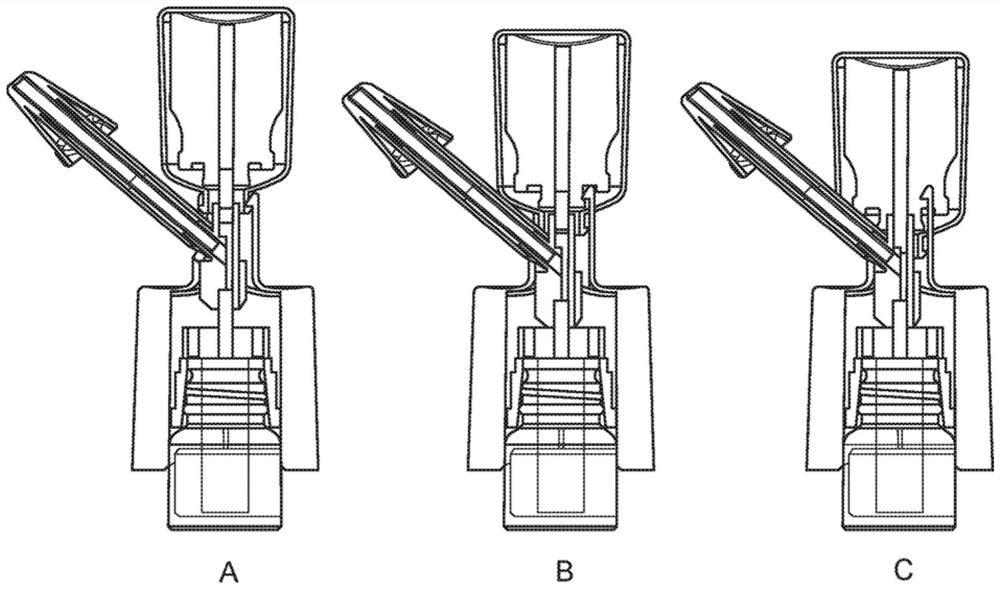
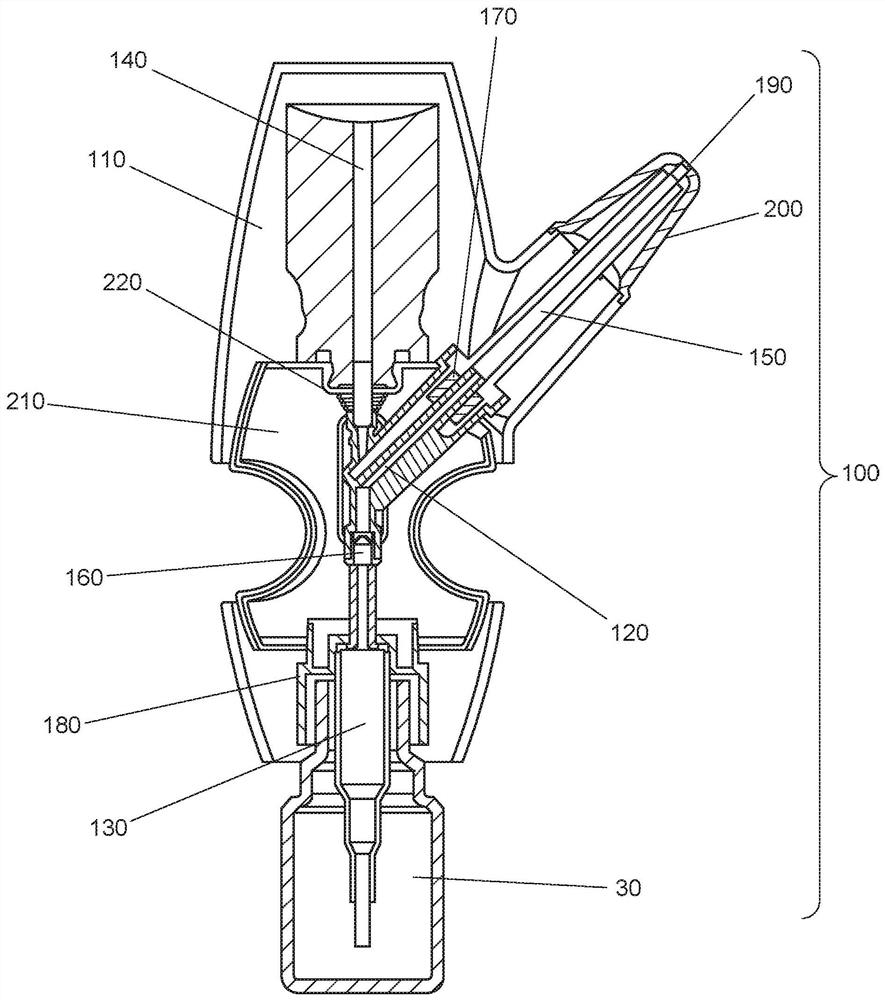
Intranasal delivery of dihydroergotamine by precision olfactory device
A technology of dihydroergotamine and dosage, which is applied in the directions of drug delivery, drug devices, and therapeutic nebulizers, etc., can solve problems such as withdrawal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0187] 5.6.2. Example 2: Phase I clinical trial
[0188] A phase I clinical trial was conducted to compare (i) the use of precision nasal delivery in healthy adult subjects Device (Impel NeuroPharma, Seattle) drug-device combination intranasally administered a single divided dose of INP104; (ii) intranasal administration nasal spray (Valeant Pharmaceuticals); and (iii) intravenous D.H.E. (Valeant Pharmaceuticals) Bioavailability of Dihydroergotamine (DHE) Mesylate.
[0189] 5.6.2.1. Study design
[0190] The study was a three-stage, three-way, randomized, open-label, single-dose, crossover, comparative bioavailability study.
[0191] In this study, 36 subjects (approximately equal numbers of males and females) were recruited and randomized. Twenty-eight subjects completed the study. Treatment assignment was randomized in a three-treatment, three-stage balanced crossover study of six sequences as follows, with a 7-day washout period between treatments:
[0192]
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com