Dihydroergotamine mesilate nasal spray
A technology of ergotamine nasal and methanesulfonic acid, which is applied in the direction of nebulizer for treatment, packaged food, packaging, etc., can solve the problems of expensive production line, incompatibility of production line, increase waste of medicinal liquid, etc. Equipment requirements, mature processing technology, and cost-saving effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1. Prescription (based on 1000 bottles, 0.5mg / spray)
[0053]
[0054] Second, the preparation process:
[0055] 1. Dosing: Weigh the raw and auxiliary materials of the prescription amount into water of 95% of the prescription amount, stir to dissolve, add water to make up the volume, and get ready.
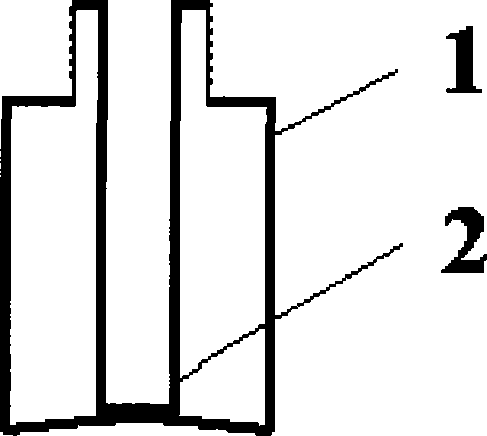
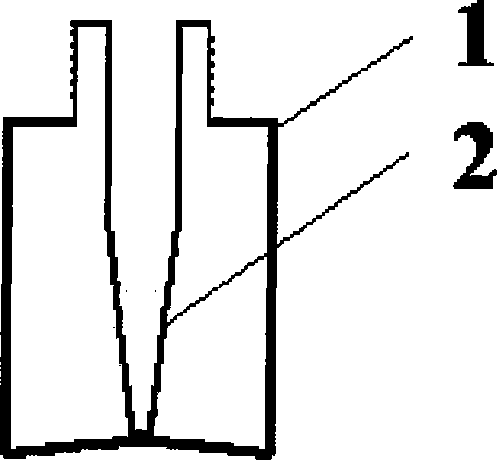
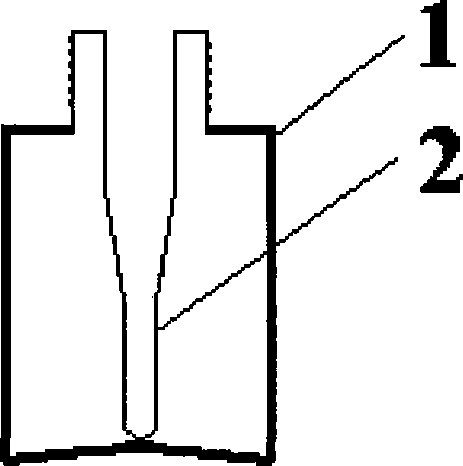
[0056] 2. Filling: Fill the dihydroergotamine mesylate aqueous solution prepared above into the attached figure 2 In the spray bottles shown, each bottle is filled with 1.0ml, filled with carbon dioxide while filling, and the spray head is tightened (the spray volume is 0.5mg / spray), that is, the final product is shown in the attached Figure 4 .
[0057] 3. Tests of effective spray times, spray uniformity and spray residual rate
[0058] The content of main ingredient per spray: same as comparative example
[0059] The preparation of reference substance solution: with comparative example
[0060] Residual main drug content in the bottle:
[0061] Take out all th...
Embodiment 2
[0073] 1. Prescription (based on 1000 bottles, 0.5mg / spray)
[0074]
[0075] Second, the preparation process:
[0076] 1. Dosing: Weigh the raw and auxiliary materials of the prescription amount into water of 95% of the prescription amount, stir to dissolve, add water to make up the volume, and get ready.
[0077] 2. Filling: Fill the dihydroergotamine mesylate aqueous solution prepared above into the attached image 3 In the spray bottles shown, each bottle is filled with 1ml, filled with carbon dioxide while filling, and the spray head is tightened (the spray volume is 0.5mg / spray), that is, the final product is shown in the attached Figure 5 .
[0078] 3. Tests of effective spray times, spray uniformity and spray residual rate
[0079] The content of main ingredient per spray: same as comparative example
[0080] The preparation of reference substance solution: with comparative example
[0081] Residual main drug content in the bottle:
[0082] Take out all the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com