Composite titanium oxide supported noble metal catalyst for hydrogen peroxide synthesis, and preparation method and application method thereof
A precious metal catalyst, composite titanium oxide technology, applied in metal/metal oxide/metal hydroxide catalyst, peroxide/peroxyhydrate/peroxyacid/superoxide/ozonide, physical/chemical process catalyst It can solve the problems of slow degradation of active intermediate anthraquinone, product pollution and high production cost, and achieve the effect of improving interface behavior, stable performance and easy control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A composite titanium oxide-supported noble metal catalyst for hydrogen peroxide synthesis, comprising:
[0032] Composite titanium oxide is used as the carrier, and the composite titanium oxide is titanium oxide compounded with a compound containing at least one element of phosphorus, sulfur, selenium, carbon, and silicon on the surface; at least one of palladium, gold, silver, and platinum is loaded on the carrier Metal.
[0033] Above, the compound is C 4 -C 20 organic phosphonic acid, C 4- C 20 thiols, C 4 -C 20 Selenate, C 4 -C 20 of sugar, C 4 -C 20 Alcohol, C 4 -C 20 Any of the silanes.
[0034] C 4 -C20 Alcohols include: furfuryl alcohol; C 4 -C 20 Organic phosphonic acids include: phenylphosphonic acid; C 4 -C 20 The thiols include: benzenethiol; C 4 -C 20 Selenates include: sodium selenite; C 4 -C 20 sugars include: glucose; C 4 -C 20 Silanes include: Octadecyltrichlorosilane.
[0035] The molar ratio of the compound to the titanium oxid...
Embodiment 2
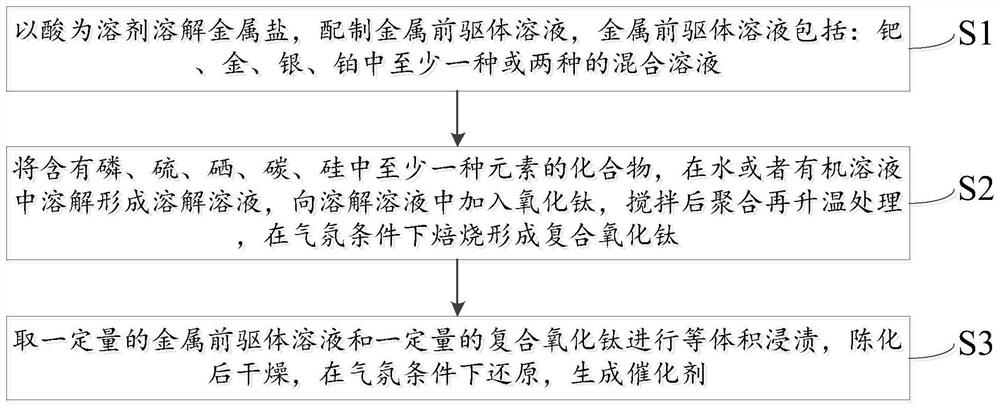
[0047] A method for preparing a composite titanium oxide-supported noble metal catalyst for hydrogen peroxide synthesis, specifically comprising:
[0048] S1. Using an acid as a solvent to dissolve a metal salt to prepare a metal precursor solution. The metal precursor solution includes: at least one or a mixed solution of two or more of palladium, gold, silver, and platinum.
[0049] Above, the acid is one or a mixture of two or more of formic acid, hydrochloric acid, nitric acid or sulfuric acid.
[0050] The concentration of the metal precursor solution is 0.001-0.5 g / mL, preferably 0.001-0.2 g / mL.
[0051] S2. A compound containing at least one element of phosphorus, sulfur, selenium, carbon, and silicon is dissolved in water or an organic solution to form a dissolved solution, and titanium oxide is added to the dissolved solution, and after stirring, it is polymerized and then heated. Bottom firing to form composite titanium oxide.
[0052] Above, the compound is C 4 -...
Embodiment 3
[0064] An application method of a composite titanium oxide-supported noble metal catalyst for hydrogen peroxide synthesis, comprising:
[0065] A composite titanium oxide-supported noble metal catalyst for hydrogen peroxide synthesis is applied to a reaction system that catalyzes the direct synthesis of hydrogen peroxide from hydrogen and oxygen.
[0066] Above-mentioned, reaction system comprises: ethanol, concentrated sulfuric acid, described catalyst are mixed, under certain temperature, pressure, in H 2 , O 2 and N 2 reaction in a mixed atmosphere. Specifically, 60ml of ethanol, 0.38ml of concentrated sulfuric acid, 50mg of catalyst, and a reaction time of 5-120min.
[0067] Specifically, the gas pressure during the reaction is 101.325kPa, the gas composition ratio is H2:O2:N2=9:15:36 (mL / min), the stirring rate is 950rpm, the reaction temperature is 10°C, and the reaction time is 1-2h.
[0068] Based on the composite titanium oxide-supported precious metal catalyst fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com