Novel immune medicine for treating solid tumors
A technology for immune drugs and tumors, applied in anti-tumor drugs, drug combinations, drug delivery, etc., can solve problems such as high incidence of tumors, endangering the health of the general public, and no breakthroughs in chemotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] This embodiment provides an immunomedicine for treating solid tumors and a preparation method thereof.
[0057] 1. The immune drugs are type B drugs, including:
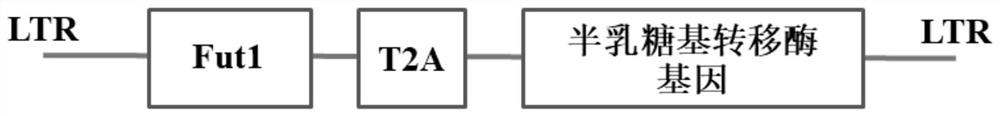
[0058] The viral expression vector pLVX-IRES-ZsGreen1 (donated by Beijing Li Keli Laboratory) that simultaneously expresses the Fut1 gene and the B blood group gene contains the nucleotide sequence SEQ ID NO.4, which includes the Fut1 gene, B blood type gene and connection sequence T2A;
[0059] The viral expression vector can express the amino acid sequence shown in SEQ ID NO.2;
[0060] 2. The preparation method of the immunomedicine is as follows:
[0061] (1) Synthetic nucleotide sequence SEQ ID NO.4;
[0062] (2) if figure 1 As shown, insert SEQ ID NO.4 into the pLVX-IRES-ZsGreen1 vector, and then package the therapeutic virus in 293T cells to obtain the new immune drug;
Embodiment 2
[0064] This embodiment provides an immunomedicine for treating solid tumors and a preparation method thereof.
[0065] 1. The immune drugs are type A drugs, including:
[0066] A virus expression vector expressing Fut1 gene and A blood group gene simultaneously, said virus expression vector comprising nucleotide sequence SEQ ID NO.3, which includes Fut1 gene, A blood group gene and connection sequence T2A; the virus expression vector can express SEQ ID NO.3 The amino acid sequence shown in ID NO.1;
[0067] 2. The preparation method of the immunomedicine is as follows:
[0068] (1) Synthetic nucleotide sequence SEQ ID NO.3;
[0069] (2) Insert SEQ ID NO.3 into the pLVX-IRES-ZsGreen1 vector, and then package the therapeutic virus in 293T cells to obtain the immune drug.
Embodiment 3
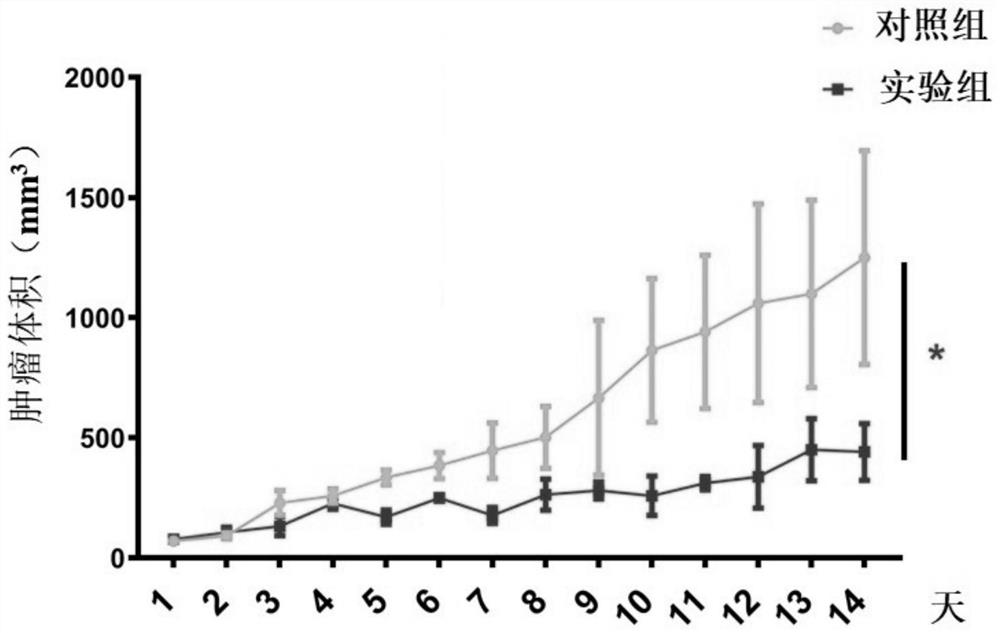
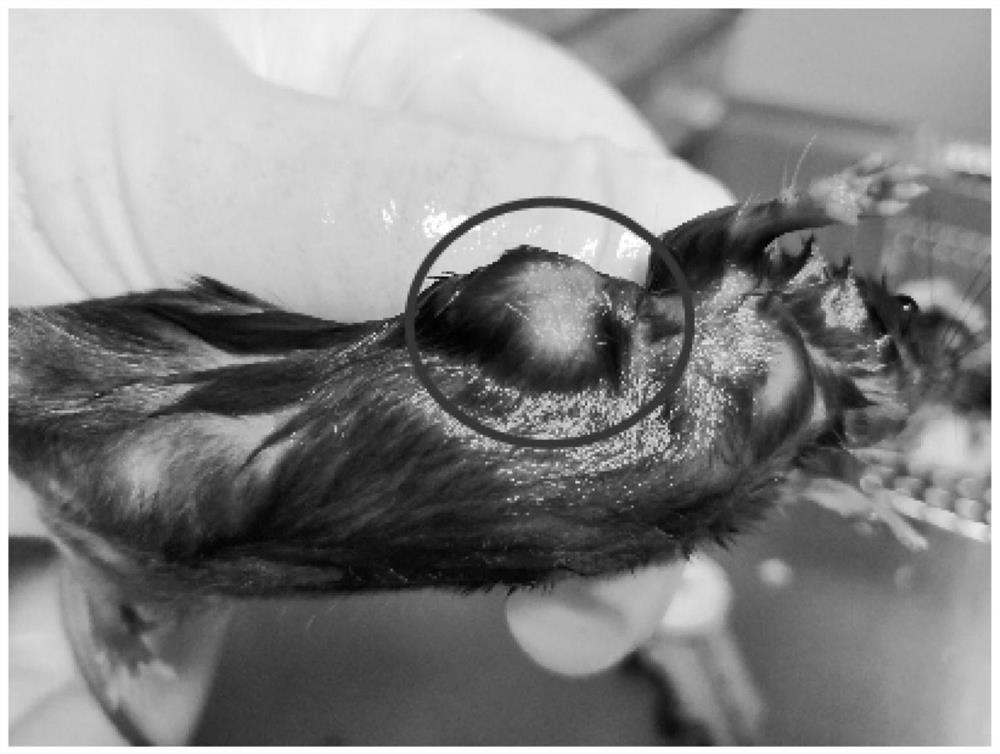
[0071] In this example, the type A drug provided in Example 2 was used to treat tumors in mice, and the therapeutic effect was observed. The specific operation steps are as follows:
[0072] The MC38 cells used in this example come from the Cell Resource Center of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences; cultured at 37°C, 5% CO 2 In a humidified atmosphere, the medium was RPMI-1640 medium (Thermo Fisher, USA) supplemented with 10% fetal bovine serum.
[0073] (1) Three weeks in advance, immunize 6 five-week-old, 20-g C57 mice (purchased from Beijing Huafukang Company) with A blood group antigen, and immunize once a week after the first immunization; under SPF conditions , mice were housed in cages, and each cage contained up to five mice at the Experimental Animal Center of Southern Medical University.
[0074] (2) Inoculate MC38 colorectal cancer cell line (2×10 5 / only) in the subcutaneous part of the mouse;
[0075] (3) After five days,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com