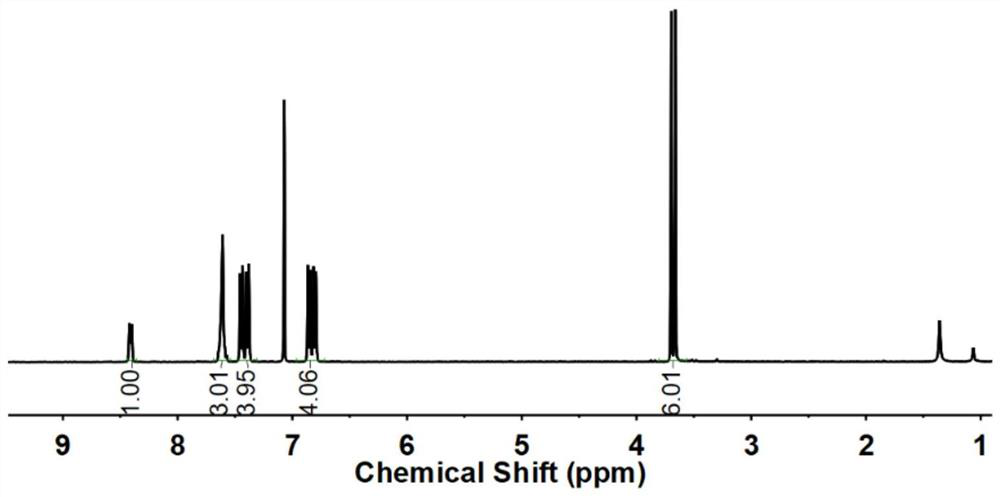
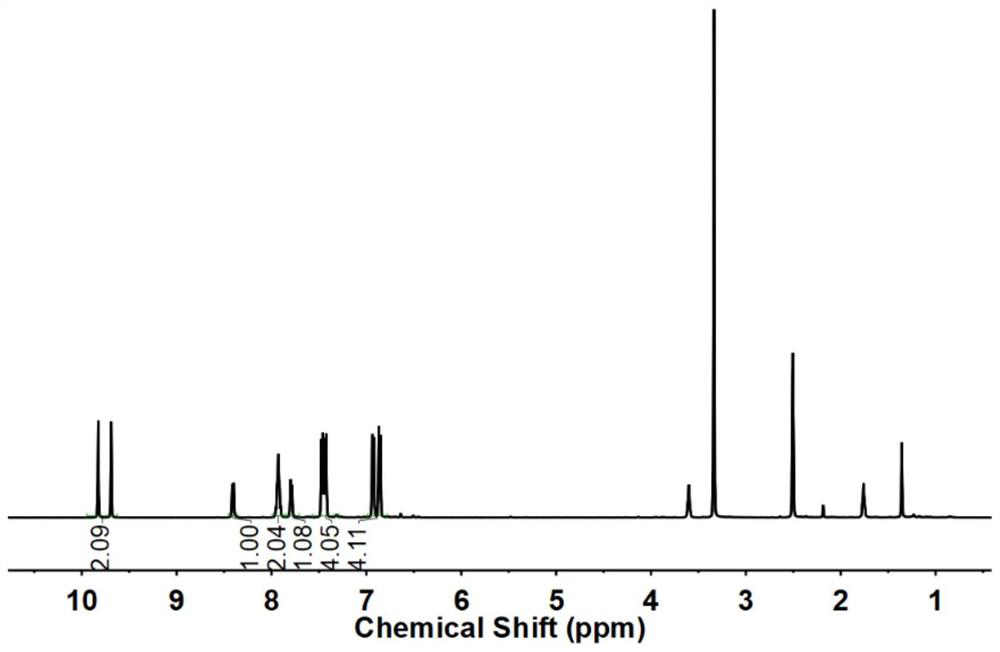
Hetero-naphthalene biphenyl bisphenol monomer and preparation method thereof, hetero-naphthalene biphenyl epoxy monomer and preparation method and application thereof, and flame-retardant epoxy resin
A technology of naphthalene biphenyl bisphenol and epoxy resin, applied in the field of bisphenol monomer, can solve problems such as poor solubility, and achieve the effects of simple post-processing, mild reaction conditions and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The embodiment of the present invention provides a kind of preparation method of xanthylbiphenyl (being phthalazinone structure) bisphenol monomer, main steps are as follows:
[0038] (1) Friedel-Crafts reaction to prepare one-sided methoxyl intermediate (MHPZ);
[0039] (2) Using MHPZ, BPM (p-bromoanisole), PNTM (1,10-phenanthroline) and CuI (cuprous iodide), the double-sided methoxy intermediate (MMPZ);
[0040] (3) MMPZ is reduced by Lewis acid to obtain dihydroxyl (bisphenol) target monomer (HHPZ);
[0041] Wherein, the synthetic routes of MHPZ, MMPZ and HHPZ are as follows:
[0042]
[0043] The specific synthesis method is as follows:
[0044] (1) The specific method for preparing MHPZ by Friedel-Crafts reaction is as follows:
[0045] In an ice-bath environment, with dichloromethane as the reaction solvent, aluminum trichloride (AlCl 3) as catalyst, reactant PA (phthalic anhydride): AS (methoxybenzene): AlCl 3 The molar ratio between them is 1:1.5:1.5. A...
Embodiment 1
[0057] Embodiment 1 provides a kind of preparation method of the bisphenol monomer containing naphthalene structure, specifically:
[0058] Step (1): The preparation process of the monomethoxyl intermediate (MHPZ) containing the naphthalene structure
[0059] In an ice-bath environment, with dichloromethane as the reaction solvent, aluminum trichloride (AlCl 3 ) as catalyst, reactant (PA:AS:AlCl 3 ) with a molar ratio of 1:1.5:1.5. After the feeding is completed, continue to stir for 1 h in an ice-bath environment, react the above mixed system at 25°C for 3 h, sink into a glacial acid bath after the reaction, and obtain a white solid powder , that is, the intermediate acid (the product of PA and AS). Dissolve the above white solid powder in a mixed solvent of sulfolane and chlorobenzene (volume ratio: 3:1), mix and stir at 110°C for 30 minutes, and then dropwise add the corresponding reactant intermediate acid (product of PA and AS) moles Fractions of hydrazine hydrate were...
Embodiment example 2
[0064] Add MHPZ and K to a three-neck flask equipped with magnetic stirring and reflux condensing device 2 CO 3 , K 2 CO 3 The amount of MHPZ is 2.5 times the molar amount of MHPZ, and the salt-forming reaction is performed at 145°C for 5 to 6 hours. After the salt-forming reaction is completed, the reaction solution is lowered to room temperature, and then the Ullmann coupling ligand PNTM, the catalyst CuI and the reaction solution are added. BPM (when feeding, the molar ratio of MHPZ, BPM, PNTM and CuI is 1:1.5-2:0.01-0.02:0.05-0.15), protected from light for 18 hours, the reaction temperature gradually increased from room temperature to 170 °C. After the reaction is finished, cool the reaction solution and sink into hot water, drain overnight to obtain a brown solid, dissolve the obtained brown solid in chloroform, suction filter the resulting filtrate, add excess anhydrous sodium sulfate to remove water, and obtain a light yellow powder after rotary evaporation solid, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com