Tobramycin raw material and related preparation impurity spectrum analysis method
A technology of tobramycin and analytical methods, applied in the direction of material separation, analytical materials, instruments, etc., can solve problems such as inappropriateness, high proficiency requirements, and deviations, and achieve good specificity, strong scalability, and responsiveness fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Solution preparation:
[0057] 1. Buffered saline solution containing ion pair reagent
[0058] Take 16 g of anhydrous sodium sulfate and 4.4 g of sodium heptane sulfonate, add 1000 ml of water to dissolve, and adjust the pH to 3.4 with glacial acetic acid.
[0059] 2. Mobile phase A
[0060] Methanol and the above-mentioned buffered saline solution containing ion pair reagent are mixed in a volume ratio of 5:95.
[0061] 3. Mobile Phase B
[0062] Methanol and the above-mentioned buffered saline solution containing ion pair reagent are mixed in a volume ratio of 40:60.
[0063] 4. Mix the reference solution
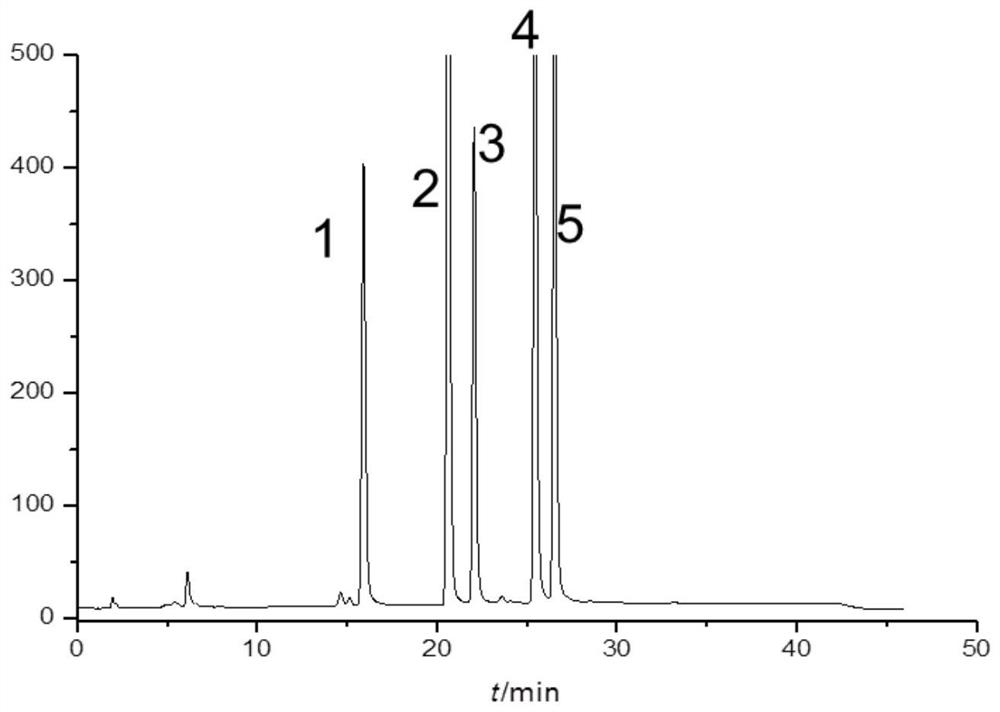
[0064] Accurately weigh tobramycin A, neomycamine, darkmycamine, kanamycin B and tobramycin reference substances, dissolve in water and dilute to a concentration of 0.1 mg / ml for each reference substance mentioned above.
[0065] 5. Alkali degradation solution
[0066] Accurately weigh the tobramycin reference substance, add water to dissolve and dilute to a concentration of 0.5...
Embodiment 2
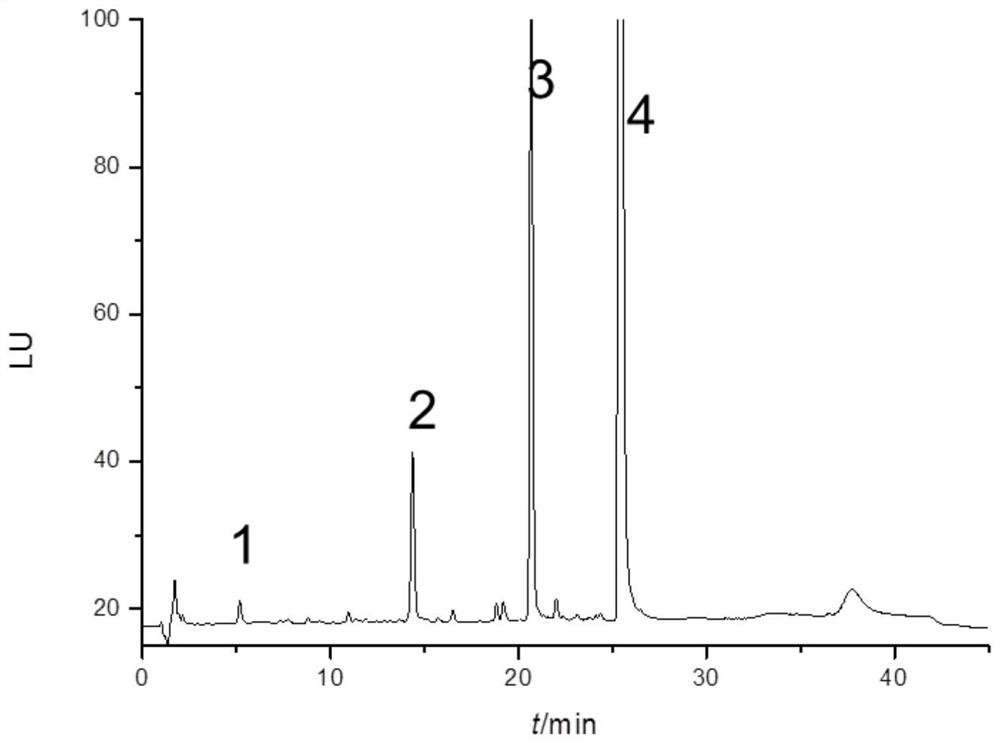
[0072] Weigh accurately tobramycin sulfate injection (source B, specification 40mg / ml), add water to dissolve and dilute to a concentration of 0.24mg / ml.
[0073] Weigh accurately tobramycin sulfate injection (source C, specification 40mg / ml), add water to dissolve and dilute to a concentration of 0.24mg / ml.
[0074] Using the same instrument and detection conditions as in implementation 1, sample injection analysis, the results are as follows Figure 4-5 Shown.
Embodiment 3
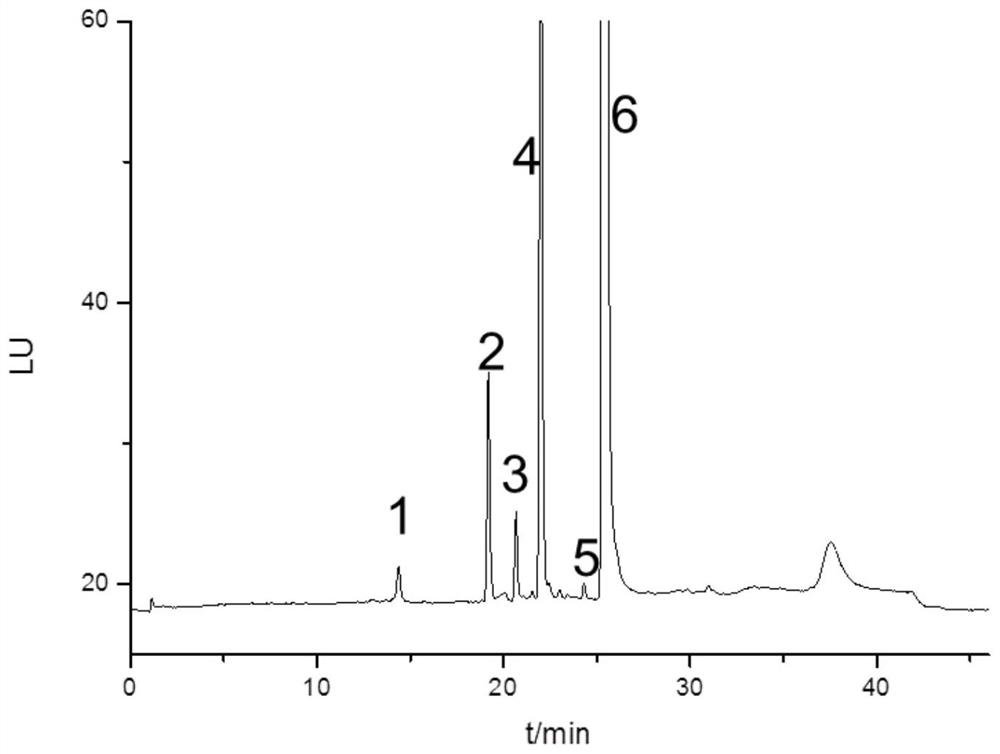
[0076] Accurately weigh tobramycin dexamethasone eye drops (source D, containing tobramycin 3mg / ml), dissolve in water and dilute to a concentration of 0.24mg / ml.
[0077] Accurately weigh loteprednol tobramycin eye drops (source E, containing tobramycin 3mg / ml), dissolve in water and dilute to a concentration of 0.24mg / ml.
[0078] Using the same instrument and detection conditions as in implementation 1, sample injection analysis, the results are as follows Figure 6-7 Shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com