Primer, probe, reagent, method and kit for normal-temperature and isothermal rapid detection of novel coronavirus SARS-CoV-2
A room temperature isothermal, coronavirus technology, applied in microorganism-based methods, biochemical equipment and methods, DNA/RNA fragments, etc., can solve problems such as unreported analysis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
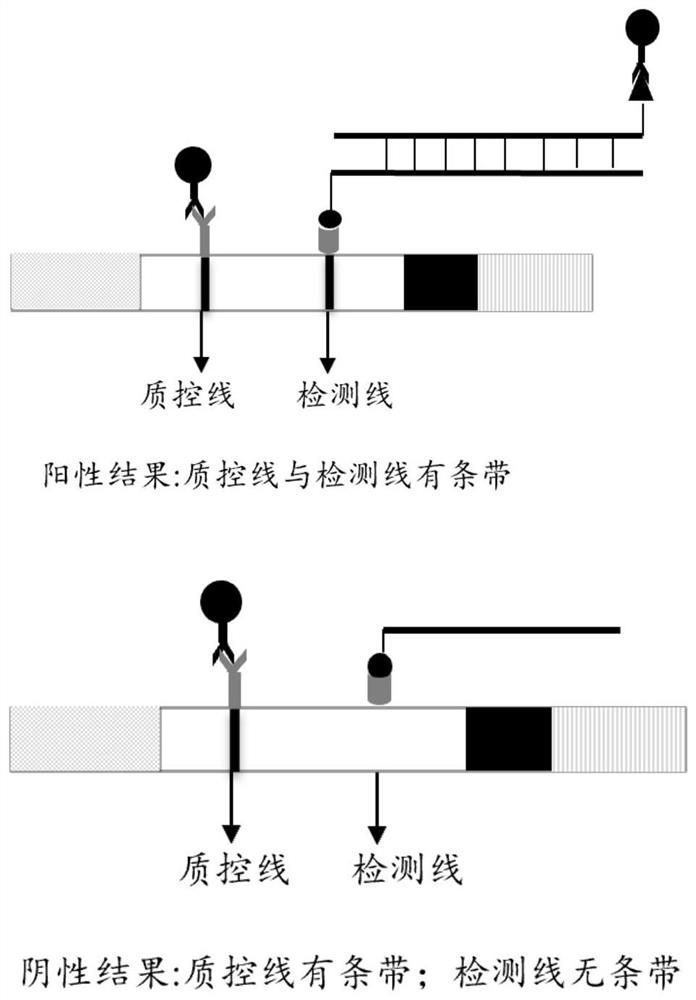
[0053] Example 1: Nucleic acid electrophoresis detection
[0054] This embodiment is used to illustrate the normal temperature and constant temperature detection carried out on the nucleic acid colloidal gold test strip.
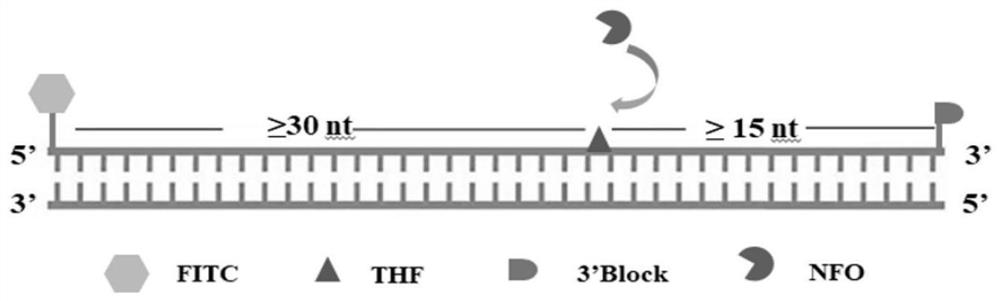
[0055] 1. According to the published SARS-CoV-2 gene sequence (accession number NC_045512.2) on NCBI GenBank, based on the design principles of primers and fluorescent probes, according to SARS-CoV-2E (Severe acute respiratory syndrome coronavirus 2) (SEQ ID NO: 4) A pair of primers (SARS-CoV-2-E-F and SARS-CoV-2-E-R) were designed, the sequence of which is shown in Table 1:
[0056] Table I
[0057]
[0058] With reference to the SARS-CoV-2 envelope protein and its 3'UTR and membrane glycoprotein gene sequence (SEQ ID NO: 4) published on GenBank, the inventors of the present application have a clear understanding of the SARS-CoV-2 envelope glycoprotein gene and its function and other information conducted a more in-depth study, and found that the envel...
Embodiment 2
[0064] Example 2: Detection of nucleic acid colloidal gold
[0065] This embodiment is used to illustrate the normal temperature and constant temperature detection carried out on the nucleic acid colloidal gold test strip.
[0066] 1. According to the published SARS-CoV-2 envelope protein and its 3'UTR and membrane glycoprotein gene sequence (SEQ ID NO: 4) on NCBI GenBank, based on the design principles of primers and fluorescent probes, according to SARS-CoV-2 A pair of primers (SARS-CoV-2-E-F and SARS-CoV-2-E-R) and a fluorescent probe (SARS -CoV-2-E-Probe), the sequence is shown in Table 2:
[0067] Table II
[0068]
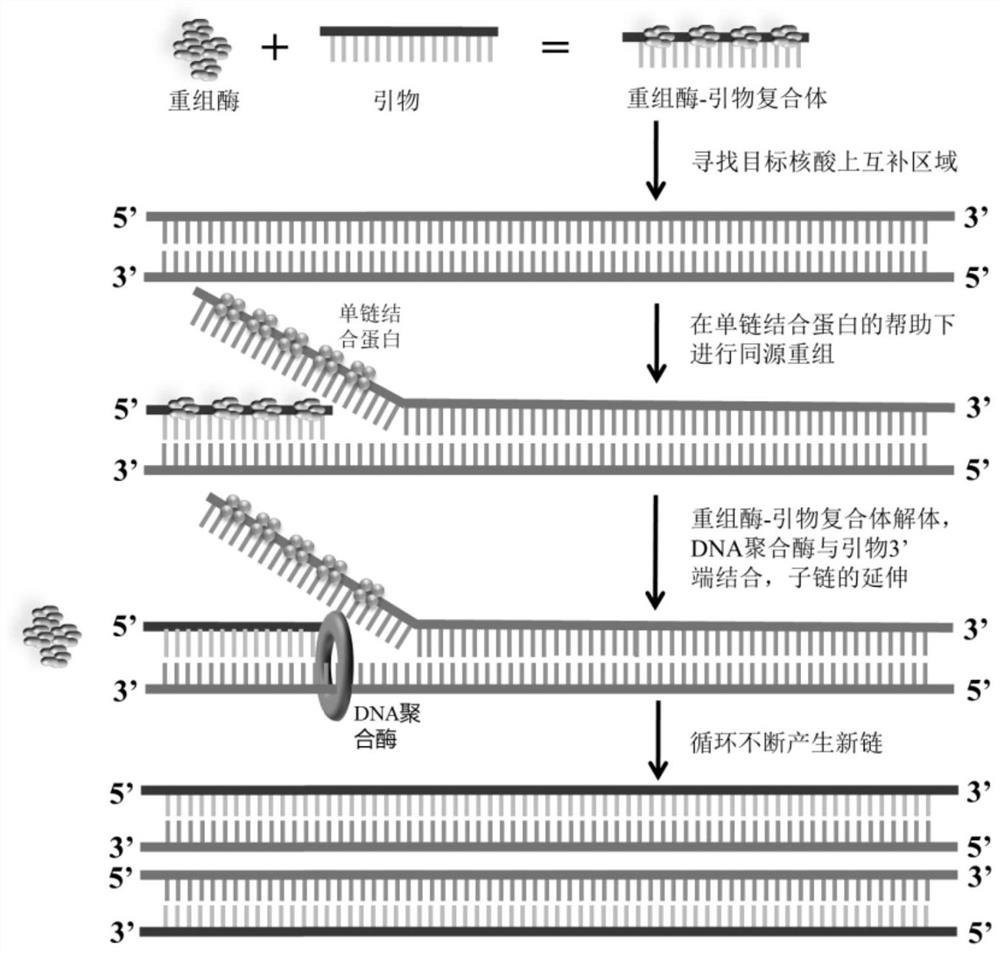
[0069] 2. The amplification reaction system is:
[0070] Table three
[0071]
[0072]
[0073] *Remarks: The lyophilized powder reagent contains the above-mentioned necessary enzymes and auxiliary components, specifically: polyethylene glycol, 4wt%; Tris, 25mM; sodium acetate, 200mM; dithiothreitol, 8mM; 10mM; creatine phosphate disodium salt, ...
Embodiment 3
[0076] Embodiment three: specificity test
[0077] For testing the specificity of the screened primers and probes, several other coronaviruses were selected for detection with primers SARS-CoV-2-E-F, SARS-CoV-2-E-R and probe SARS-CoV-2-E-Probe , verify whether there will be non-specific amplification, and interpret false positive results. Select MERS-CoV (NCBI accession number NC_019843.3, provided by the China Center for Disease Control for scientific research use, and the sequence corresponds to NCBI) and SARS-CoV (NCBI accession number NC_004718.3, provided by the China Center for Disease Control for scientific research use, Sequences correspond to NCBI) for comparison.
[0078] Experimental procedure: 1) Add 29.4μL A buffer (A buffer: 20mM tris-HCl (pH 8.5, 25°C), 150mM KCl, 5v / v% PEG, 1v / v% TRITON-X100);
[0079] 2) Add 2 μL of upstream primer, 2 μL of downstream primer and 0.6 μL of probe to each reaction tube (the concentration of primer and probe is 10 uM);
[0080...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com