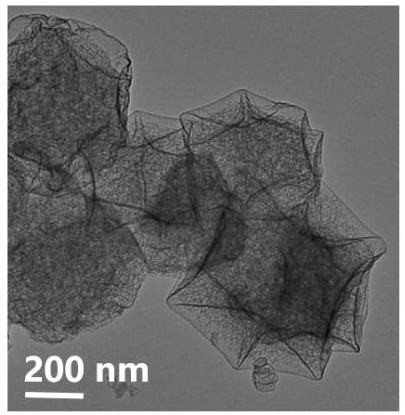
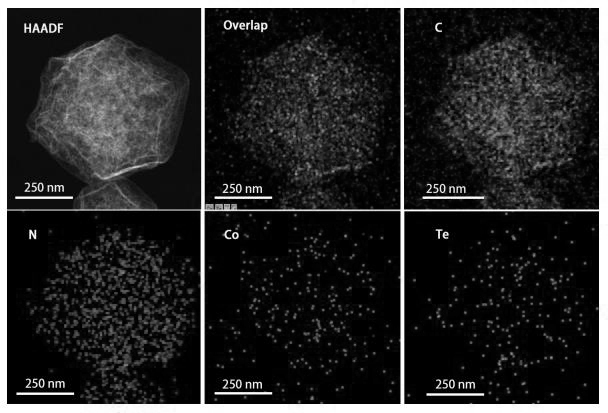
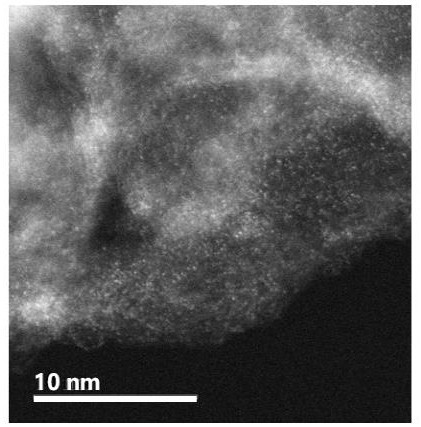
Preparation method and application method of cobalt-tellurium diatomic site catalyst
A diatomic and catalyst technology, applied in the field of electrocatalysis, can solve the problems of restricting large-scale application, high cost, and low reserves, and achieve the effect of excellent multifunctional catalytic performance, low cost, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 5.58g Zn(NO 3 ) 2 ·6H 2 O and 0.5 g of Te powder were dissolved in 150 mL of methanol to form solution A. 6.16 g of dimethylimidazole was dissolved in 150 mL of methanol to form clear solution B. Then, slowly pour solution B into solution A. After mixing and stirring at room temperature for 24 h, the precipitate was centrifuged, washed several times with methanol, and then dried at 60 °C to obtain a gray solid powder, which was recorded as Te 1 @ZIF-8.
[0037] The above Te 1 @ZIF-8 was dissolved in 50mL DMF to form solution C, 50mg tetraphenylporphyrin cobalt was dissolved in 50mL DMF to form solution D, then solution D was slowly added to solution C, mixed and stirred at room temperature for etching After 24 h of ion exchange, the precipitate was centrifuged, washed several times with ethanol, and then dried at 60 °C to obtain a beige solid powder, which was denoted as Co 0 -Te 1 @ZIF-8.
[0038] The above Co 0 -Te 1 @ZIF-8 was pyrolyzed at 920°C for 2 hour...
Embodiment 2
[0040] 5.58g Zn(NO 3 ) 2 ·6H 2O and 0.5 g of Te powder were dissolved in 150 mL of methanol to form solution A. 6.16 g of dimethylimidazole was dissolved in 150 mL of methanol to form clear solution B. Then, slowly pour solution B into solution A. After mixing and stirring at room temperature for 24 h, the precipitate was centrifuged, washed several times with methanol, and then dried at 60 °C to obtain a gray solid powder, which was recorded as Te 1 @ZIF-8.
[0041] The above Te 1 @ZIF-8 was dissolved in 50ml DMF to form solution C, 100mg tetraphenylporphyrin cobalt was dissolved in 50ml DMF to form solution D, then solution D was slowly added to solution C, mixed and stirred at room temperature for etching After 24 h of ion exchange, the precipitate was centrifuged, washed several times with ethanol, and then dried at 60 °C to obtain a gray-brown solid powder, which was recorded as Co-Te 1 @ZIF-8.
[0042] The above Co-Te 1 @ZIF-8 was pyrolyzed at 920°C for 2 hours ...
Embodiment 3
[0044] 5.58g Zn(NO 3 ) 2 ·6H 2 O and 1 g of Te powder were dissolved in 150 mL of methanol to form solution A. 6.16 g of dimethylimidazole was dissolved in 150 mL of methanol to form clear solution B. Then, slowly pour solution B into solution A. After mixing and stirring at room temperature for 24 h, the precipitate was centrifuged, washed several times with methanol, and then dried at 60 °C to obtain a gray solid powder, which was recorded as Te 2 @ZIF-8.
[0045] The above Te 2 @ZIF-8 was dissolved in 50ml DMF to form solution C, 100mg tetraphenylporphyrin cobalt was dissolved in 50ml DMF to form solution D, then solution D was slowly added to solution C, mixed and stirred at room temperature for etching After 24 h of ion exchange, the precipitate was centrifuged, washed several times with ethanol, and then dried at 60 °C to obtain a gray-brown solid powder, which was recorded as Co-Te 2 @ZIF-8.
[0046] The above Co-Te 2 @ZIF-8 was pyrolyzed at 920°C for 2 hours i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com