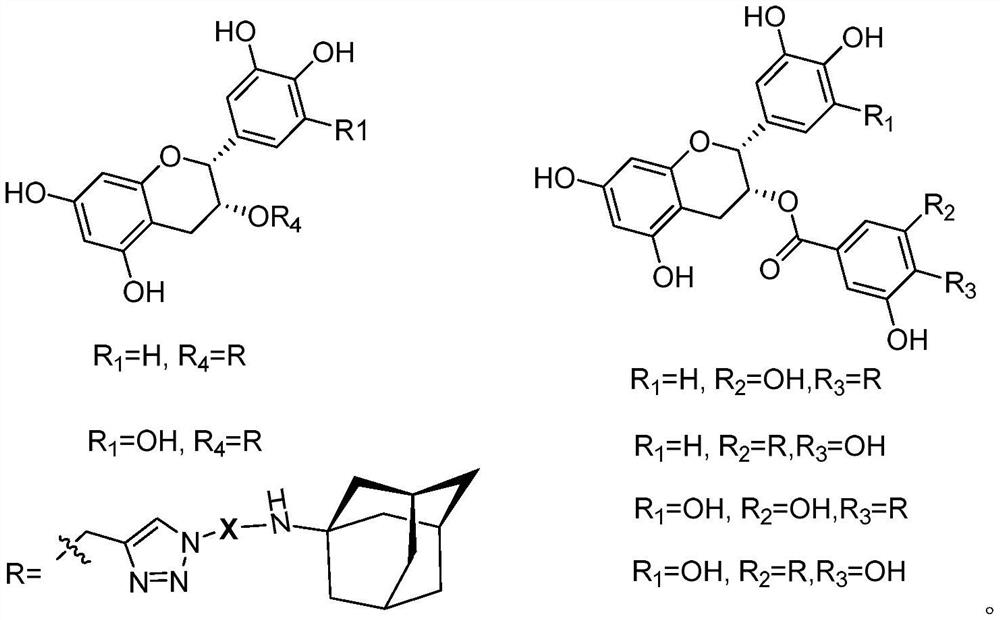
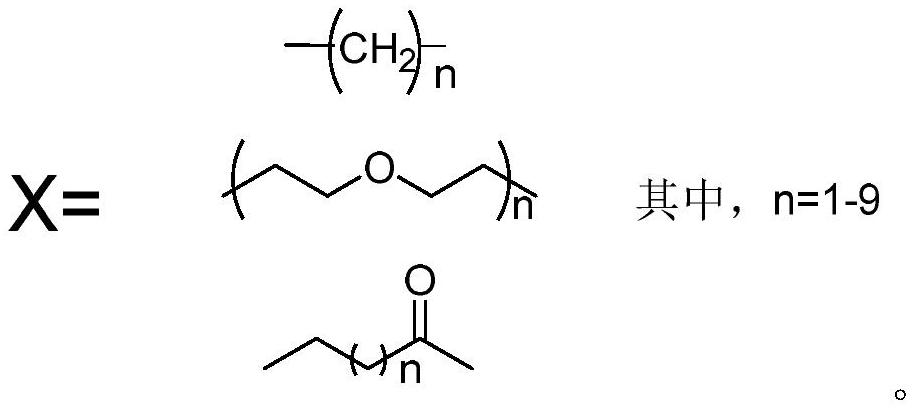
Catechin-amantadine conjugate and its preparation method and application
A technology of amantadine and catechin, applied in organic chemistry, antiviral agents, etc., can solve the problems of short half-life, low utilization rate, poor stability, etc., and achieve the effect of novel structure, simple route, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
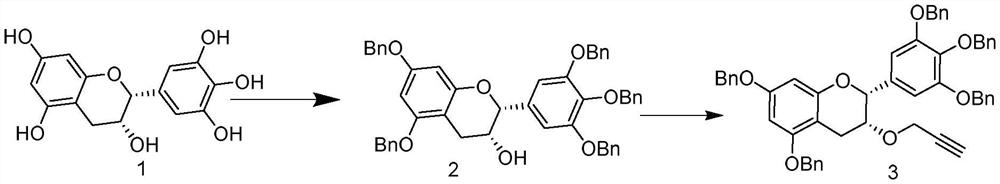
[0024] Example 1: Preparation of 5,7,3',4',5'-penta-O-benzyl epigallocatechin (2)
[0025] Epigallocatechin (EGC, 3.06g, 10mmol) was dissolved in DMF (50mL), the temperature was lowered to 0°C, potassium carbonate (10.425g, 75mmol) was added, and benzyl bromide (10.2g, 60mmol ), filled with nitrogen, reacted at room temperature, TLC monitored that the reaction was complete, added 300mL water, dichloromethane 100mL extracted 3 times, anhydrous anhydrous NaSO 4 Dry, filter and concentrate. The concentrate was separated and purified by column chromatography to obtain compound 2 (85%). 1 H NMR (600MHz, CDCl 3 )δ7.45–7.27(m,25H),6.81(s,2H),6.28(s,2H),5.13(s,4H),5.06(s,2H),5.02(s,4H),4.89(s ,1H),4.21(s,1H),3.01(d,J=17.1Hz,1H),2.93(dd,J=17.1,4.5Hz,1H). 13 C NMR (151MHz, CDCl 3 )δ158.78,158.28,155.07,153.00,138.34,137.79,136.96,136.89,133.71,128.58,128.54,128.51,128.47,128.46,128.42,128.14,127.97,127.89,127.87,127.79,127.54,127.50,127.20,127.15,106.14 , 100.96, 94.70, 94.13, 78....
Embodiment 2
[0026] Example 2: Preparation of 3-propargyl-5,7,3',4',5'-penta-O-benzyl epigallocatechin (3)
[0027] Add THF (15mL) and (1.05g, 3mmol) in a 200mL round bottom flask, add NaH (60%, 0.144g, 3.6mmol) in batches, after stirring at room temperature for 2h, add propargyl bromide (3.3mmol) dropwise at room temperature ) in THF (15 mL). After the dropwise addition was completed, the reaction was carried out at 65°C for 48 hours. After cooling down to room temperature, ice water and ethyl acetate (30 mL) were added, the organic layer was separated, and the aqueous layer was extracted twice with ethyl acetate. The combined organic layers were washed with NaHCO 3 Aqueous and NaCl aqueous washes, anhydrous NaSO 4 Dry and concentrate. The concentrate was separated and purified by column chromatography to obtain compound 3 (92%). 1 H NMR (600MHz, Chloroform-d) δ7.45–7.29(m,21H),7.28–7.24(m,4H),6.86(s,2H),6.30–6.24(m,2H),5.13(d,J =1.8Hz,4H),5.07(d,J=4.4Hz,2H),5.03(s,2H),5.00(s,2H),4.9...
Embodiment 3
[0028] Embodiment 3: the synthesis of azidoacetic acid derivatives:
[0029]
[0030] Compound bromoacetic acid (6.8mmol) and NaN 3 (1.326g, 20.4mmol) was added to DMF (15mL), stirred at 60°C for 30h, added water (30mL), adjusted pH=2 with 1M hydrochloric acid, extracted with ether (3*30mL), and the extract was washed with saturated NaHCO 3 (3*20 mL) and water (3*30 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give azidoacetic acid.
[0031] Azidoacetic acid, yield 89.2%, 1 H NMR (400MHz, CDCl 3 )δ11.68(s,1H),3.97(s,2H); 13 CNMR (100MHz, CDCl 3 )δ174.5,50.0.ESI-MS: m / z[M+H + ]: 102.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com