Synthesis method of aromatic benzyl ketone
A technology of aromatic benzyl ketone and synthesis method, applied in the field of synthesis of aromatic benzyl ketone, can solve problems such as hidden danger, waste safety, pollution, etc., and achieve the effects of promoting reaction temperature, reducing dosage, and promoting self-oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
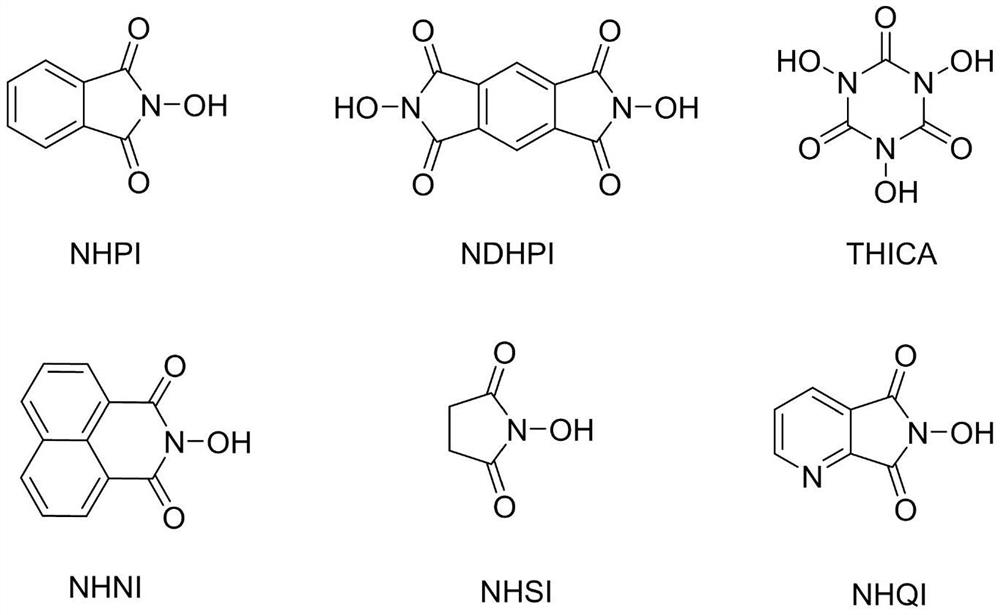
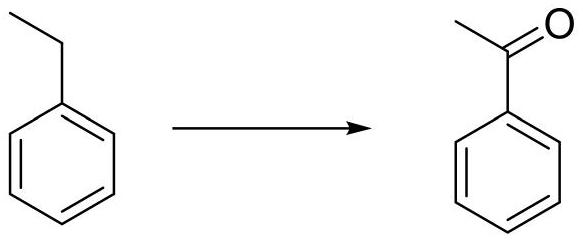
[0038]A round bottom flask equipped with a mechanical stirring bar and a reflux condenser was charged with 42.5 g of ethylbenzene, 4.8 g of ferric nitrate nonahydrate, 3.2 g of NHPI, 200 mL of acetic acid. After the addition, the temperature of the oil bath was raised to 100° C., and the reaction was carried out under open-open stirring for 12 hours. Gas chromatography detection showed that the conversion rate of ethylbenzene was 98%, and the reaction was stopped. After the acetic acid in the reaction system was distilled off under reduced pressure, 45.2 g of the product acetophenone (colorless liquid) was obtained by distillation under reduced pressure, with a yield of 94%.
[0039] Product NMR identification data:
[0040] 1 H NMR (500MHz, Chloroform-d) δ7.93-7.91 (m, 2H), 7.52 (tt, J=7.5, 1.5Hz, 1H), 7.43–7.40 (m, 2H), 2.55 (s, 3H).
[0041] 13 C NMR (125MHz, Chloroform-d) δ198.06, 137.09, 133.09, 128.55, 128.28, 26.54.
Embodiment 2
[0043]
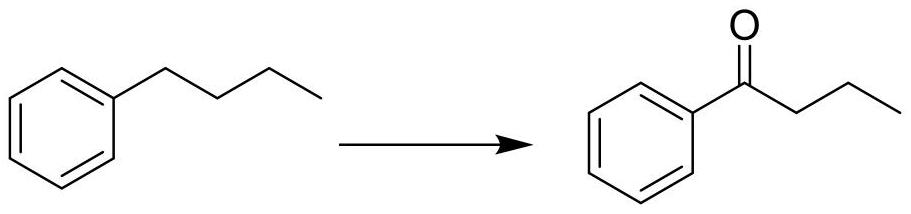
[0044] A round bottom flask equipped with a magnetic stirring bar and a reflux condenser was charged with 5.3 g of butylbenzene, 0.4 g of ferric nitrate nonahydrate, 0.3 g of NHPI, and 30 mL of acetonitrile. After the addition, put on the three-way valve and connect the oxygen ball, and perform three ventilations under the action of the air pump to replace the air in the reaction system with oxygen, raise the temperature of the oil bath to 100°C, and react for 15 hours under stirring and reflux Afterwards, gas chromatographic detection showed that the conversion rate of butylbenzene was 91%, and the reaction was stopped. After the solvent in the reaction system was distilled off under reduced pressure, it was separated by column chromatography (petroleum ether / ethyl acetate=100:1-30:1) to obtain 5.28 g of the product butyrophenone (colorless liquid), with a yield of 89% .
[0045] Product NMR identification data:
[0046] 1 H NMR (500MHz, Chloroform-d) δ7.95-7.9...
Embodiment 3
[0049]
[0050] A round bottom flask equipped with a mechanical stirring bar and a reflux condenser was charged with 6.6 g of diphenylmethane, 0.4 g of ferric nitrate nonahydrate, 0.3 g of NHPI, 20 mL of benzonitrile. After the addition, put on the three-way valve to connect the oxygen ball, and perform three air changes under the action of the air pump to replace the air in the reaction system with oxygen, and raise the temperature of the oil bath to 90 °C. After 12 hours of reaction, the gas phase Chromatographic detection showed that the conversion rate of diphenylmethane was 100%, and the reaction was stopped. After the benzonitrile in the reaction system was distilled off under reduced pressure, it was separated by column chromatography (petroleum ether / ethyl acetate=100:1-50:1) to obtain 7.13 g of the product benzophenone (white solid, melting point 47-50:1). 48°C), yield 99%.
[0051] Product NMR identification data:
[0052] 1 H NMR (500MHz, Chloroform-d) δ7.88–7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com