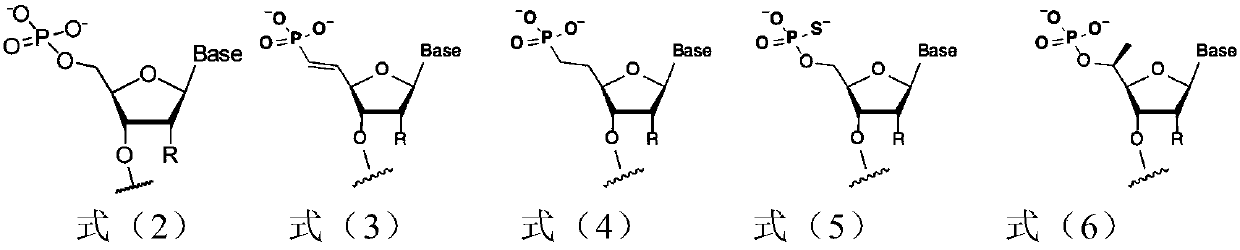
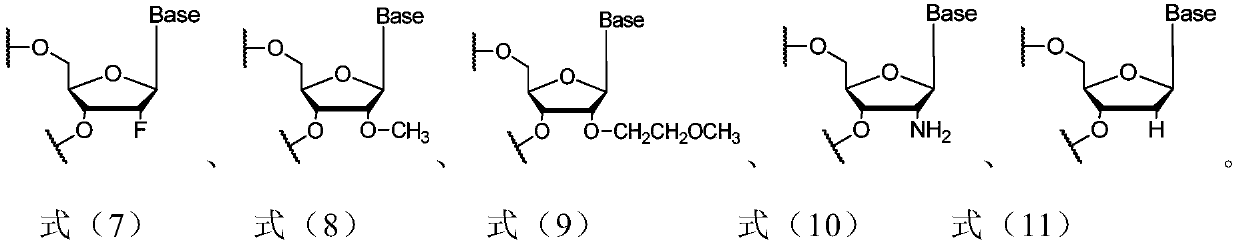
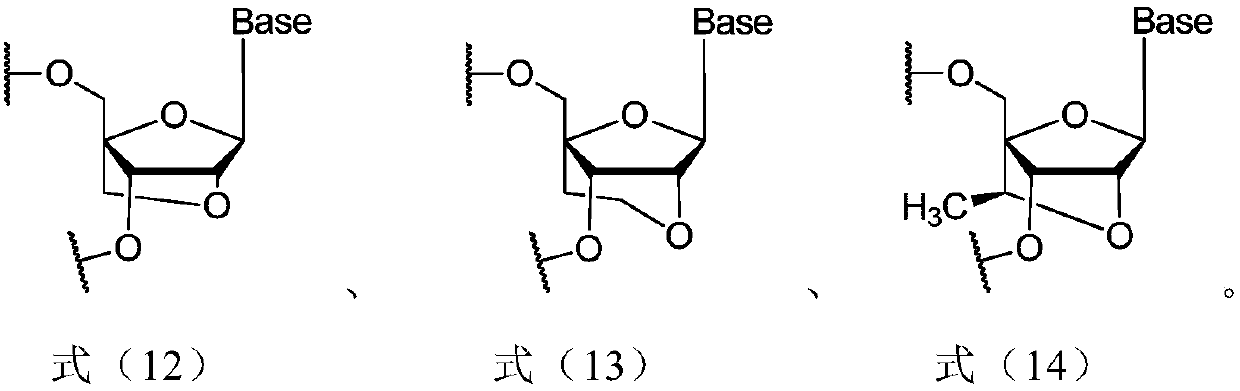
siRNA for inhibiting expression of CTGF gene, pharmaceutical composition containing siRNA and use of pharmaceutical composition
A gene expression and base technology, applied in the field of nucleic acid and pharmaceutical composition, can solve the problems of poor stability of siRNA activity, slow progress, slow progress of drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0236] Unless otherwise specified, the reagents and media used in the following examples are commercially available, and the nucleic acid electrophoresis, real-time PCR and other operations used are all referring to the methods described in Molecular Cloning (Cold Spring Harbor Laboratory Press (1989)) conduct.
[0237] Hela cells were provided by the Laboratory of Nucleic Acid Technology, Institute of Molecular Medicine, Peking University, using 20% fetal bovine serum (FBS, Hyclone Company) and 0.2 volume% penicillin-streptomycin (Penicillin-Streptomycin, Gibco, Invitrogen Company) Cells were cultured in complete DMEM medium (Hyclone Company) at 37 °C in 5% CO 2 / 95% air incubator.
[0238] Unless otherwise stated, the proportions of reagents provided below are all calculated by volume ratio (v / v).
preparation example 1
[0239] Preparation example 1 Synthetic siRNA sequence
[0240] In siRNA synthesis, unless otherwise specified, the nucleoside monomer (nucleoside monomer) refers to the modified nucleoside phosphoramidite used in the solid-phase synthesis of phosphoramidite according to the type and sequence of nucleotides in the siRNA to be prepared Monomers (modified RNA phosphoramidites). Phosphoramidite solid phase synthesis is a method used in RNA synthesis well known to those skilled in the art. Unless otherwise specified, the nucleoside monomers used are commercially available.
[0241] (1-a) Solid phase phosphoramidite method
[0242] The siRNA sequences listed in Table 2 were obtained by the solid-phase phosphoramidite method.
[0243] For the sense strand, use a universal solid phase carrier (UnyLinker TM loaded HL SolidSupports (Kinovate Life Sciences company) starts the cycle, and connects nucleoside monomers one by one from the 3'-5' direction according to the sequence of nu...
experiment example 1
[0283] Experimental example 1 Detection of the inhibitory efficiency of siRNA on CTGF mRNA expression in Hela cells.
[0284] Using Lipofectamine TM 2000 The siRNA obtained in Preparation Example 1 was transfected into Hela cells respectively, and the final concentration of siRNA was 50 nM. Each siRNA was transfected in triplicate wells. Cells without any siRNA treatment served as blank control.
[0285] The expression levels of CTGF mRNA in Hela cells transfected with each siRNA were detected by real-time fluorescent quantitative PCR (Quantitative Real-Time PCR). The specific steps are: after culturing the transfected cells for 24 hours, use Trizol (Thermo Fisher Company) to extract the total RNA in the cells according to the standard operation procedure of total RNA extraction; take 1 μg of total RNA respectively, and use a reverse transcription kit (Promega Company) , Cat. No. A3500) were reverse-transcribed to obtain cDNA according to the operation method in the manual....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com