Energy absorption method based on dynamic polymer
A polymer, dynamic technology for energy absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
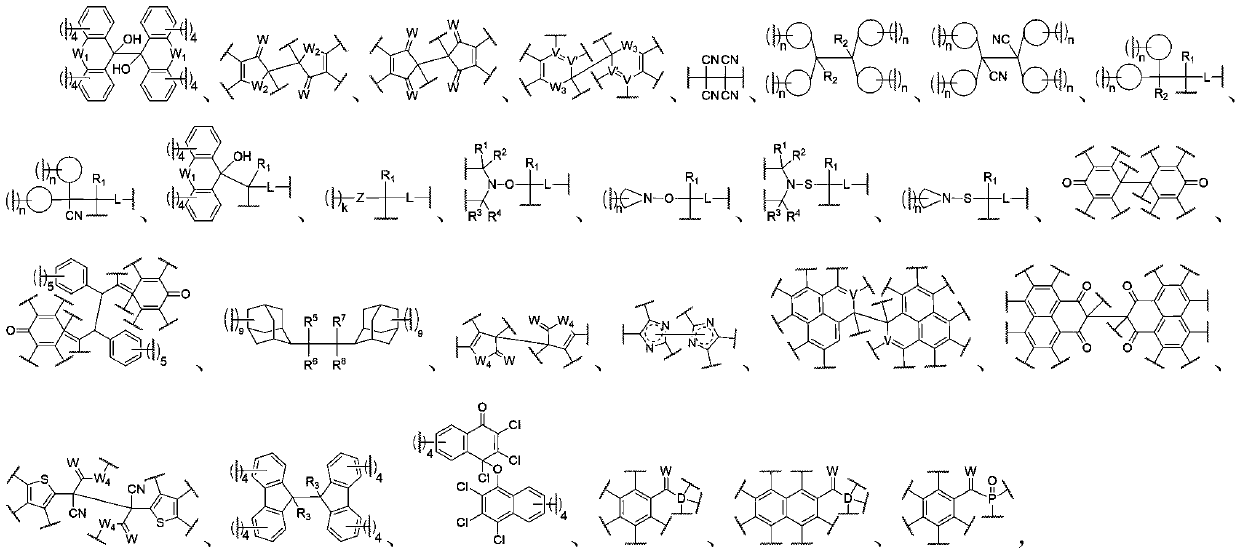
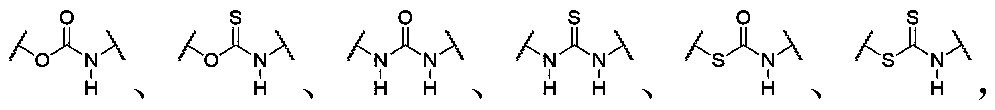
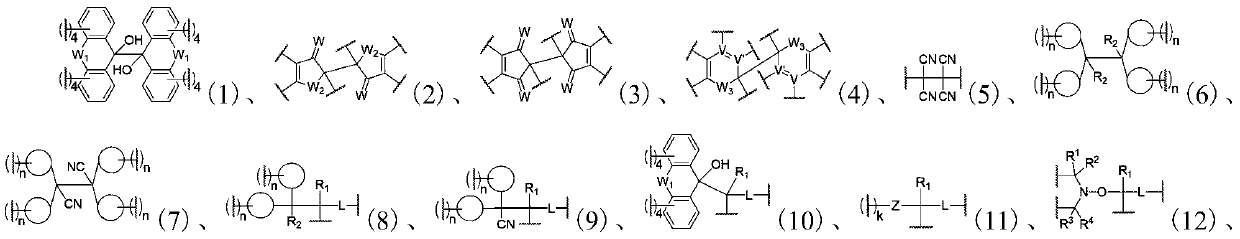
[0337] In the present invention, each raw material / component of the dynamic polymer may have one, two or more than two glass transition temperatures, or may not have a glass transition temperature. Among them, in a preferred embodiment of the present invention, at least one of the glass transition temperatures of the raw materials / components of the dynamic polymer is higher than 100°C, more preferably all of them are higher than 100°C, which is convenient for preparation and has good dimensional stability . A high temperature resistant energy-absorbing material with excellent mechanical properties; in another preferred embodiment of the present invention, at least one of the raw materials / components of the dynamic polymer has a glass transition temperature between 25°C and 100°C, More preferably all of them are between 25°C and 100°C, which results in an energy-absorbing material containing at least one glass transition temperature between 25°C and 100°C; in another preferred e...
Embodiment 1
[0356]
[0357] Use hydroxy-terminated polyethylene glycol (PEG-2000) as the reactant and toluene as the solvent to chlorinate the terminal hydroxy group with thionyl chloride at 80°C; then use DMF as the solvent to react it with sodium azide, Azido-terminated polyethylene glycols are obtained. Take 1 molar equivalent of compound (a), 2 molar equivalents of 5-hexynoic acid, 0.5 molar single-quantity 4-dimethylaminopyridine, and 3 molar equivalents of dicyclohexylcarbodiimide, place them in a reaction vessel, and use an appropriate amount of two Chloromethane was dissolved, and then stirred at room temperature for 16 hours to obtain compound (b). Take 5 molar equivalents of compound (b), 5 molar equivalents of terminal azidopolyethylene glycol, 10 molar equivalents of cuprous bromide, and 10 molar equivalents of pentamethyldiethylenetriamine, place them in a reaction vessel, and dissolve them with tetrahydrofuran As a solvent, under a nitrogen atmosphere, stir the reaction ...
Embodiment 2
[0359]
[0360] Using methyl 2-bromopropionate as the initiator, cuprous bromide and pentamethyldiethylenetriamine as the catalyst system, bromine mono-terminated polymethacrylic acid was prepared from methyl methacrylate by ATRP reaction Methyl ester; then using tetrahydrofuran as a solvent, the above-mentioned polymethyl methacrylate and excess sodium azide were reacted at room temperature for 24 hours to obtain azido-single-terminated polymethyl methacrylate; take 15 molar equivalents of azide Polymethyl methacrylate, 7.5 molar equivalents of diacetylenyl compound (a), 75 molar equivalents of pentamethyldiethylenetriamine, are placed in a reaction vessel, dissolved with an appropriate amount of tetrahydrofuran, and nitrogen gas Bubble deoxygenation for 30 minutes, then add 60 molar equivalents of copper powder and 15 molar equivalents of cuprous bromide, under nitrogen atmosphere, stir and react at room temperature for 5 hours, pour the resulting product into a mold, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Elastic modulus | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com