Demulsification method of recombinant protein oil emulsion subunit vaccine
A technology of subunit vaccines and recombinant antigenic proteins, which is applied in the direction of vaccines, veterinary vaccines, biochemical equipment and methods, etc., can solve the problems that the positive control of the test product cannot be established, the range value of the endotoxin content cannot be detected, etc., and achieve The method is simple, the effect is good, and the antigen recovery rate is high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1 Establishment of Demulsification Parameters of Ultrasonic Demulsification Demulsification Method
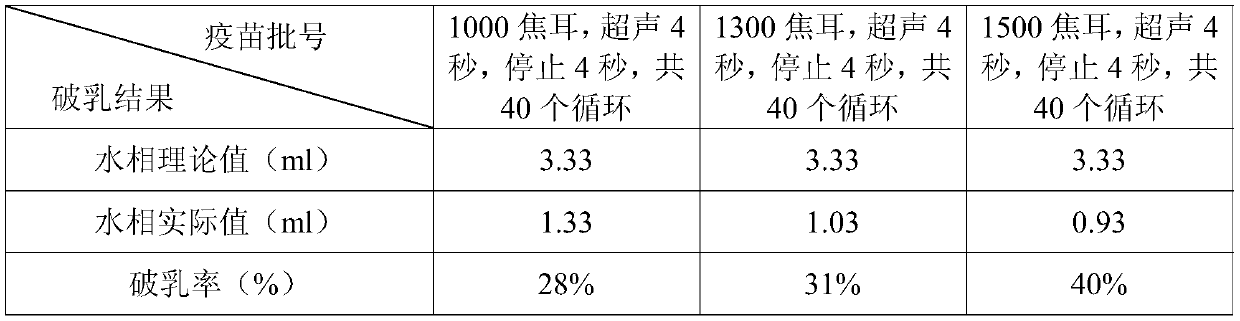
[0017] 1.1 Establishment of Ultrasonic Demulsification Parameters Take 10ml of genetically engineered subunit vaccine for chicken infectious bursal disease, and use the following three conditions for demulsification: 1000 joules, ultrasound for 4 seconds, stop for 4 seconds, a total of 40 cycles; 1300 Joule, ultrasonic for 4 seconds, stop for 4 seconds, a total of 40 cycles; 1500 joules, ultrasonic for 4 seconds, stop for 4 seconds, a total of 40 cycles; after the ultrasonic, centrifuge at 8000 rpm / min for 10 min at room temperature to obtain the aqueous phase.
[0018] 1.2 Theoretical value of the water phase of the vaccine after demulsification The ratio of the water phase to the oil phase is 1:2 when the chicken infectious bursal disease genetically engineered subunit vaccine is emulsified, so the theoretical value of the water phase after demulsification shoul...
Embodiment 2
[0022] Example 2 Preparation of Chicken Infectious Bursal Disease Genetic Engineering Subunit Vaccine and Its Emulsification Method
[0023] 2.1 Preparation of Chicken Infectious Bursal Disease Virus VP2 Protein The recombinant Escherichia coli was cultured and induced to express in a fermenter. After the fermentation, the collected wet bacteria were resuspended with an appropriate amount of PBS solution, and used at 4°C Ultrasonic crushing. The crushed bacterial liquid was centrifuged at 12000r / min for 15 minutes, and the supernatant was collected. Protein purification by neutral salt salting out method. Triton X-114 and allantoin were used to remove endotoxin to obtain VP2 protein solution.
[0024] 2.2 Detection of VP2 protein content The agar diffusion test was performed on the supernatant after breaking the bacteria, and the AGP titer of the expression product VP2 in the supernatant was recorded. It is 1:32.
[0025] 2.3 Inactivation Put the VP2 protein solution in a ...
Embodiment 3
[0053] Example 3: Using two demulsification methods to detect water phase endotoxin content after oil emulsion vaccine demulsification
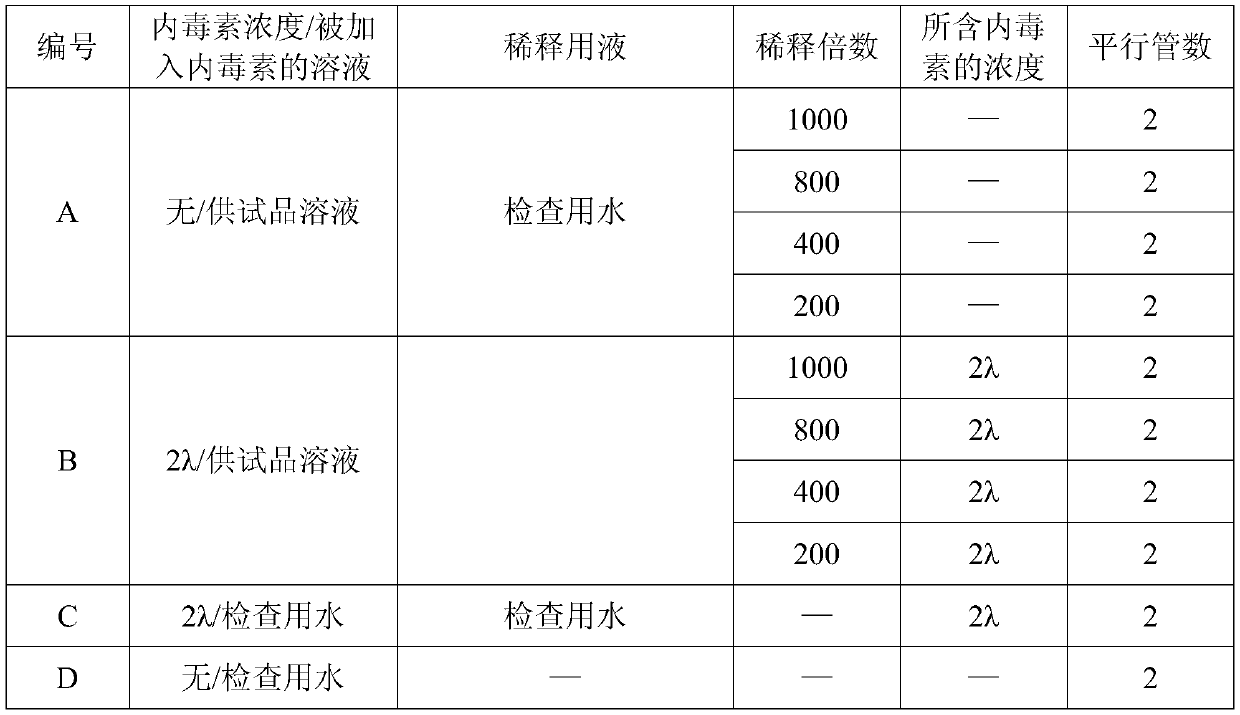
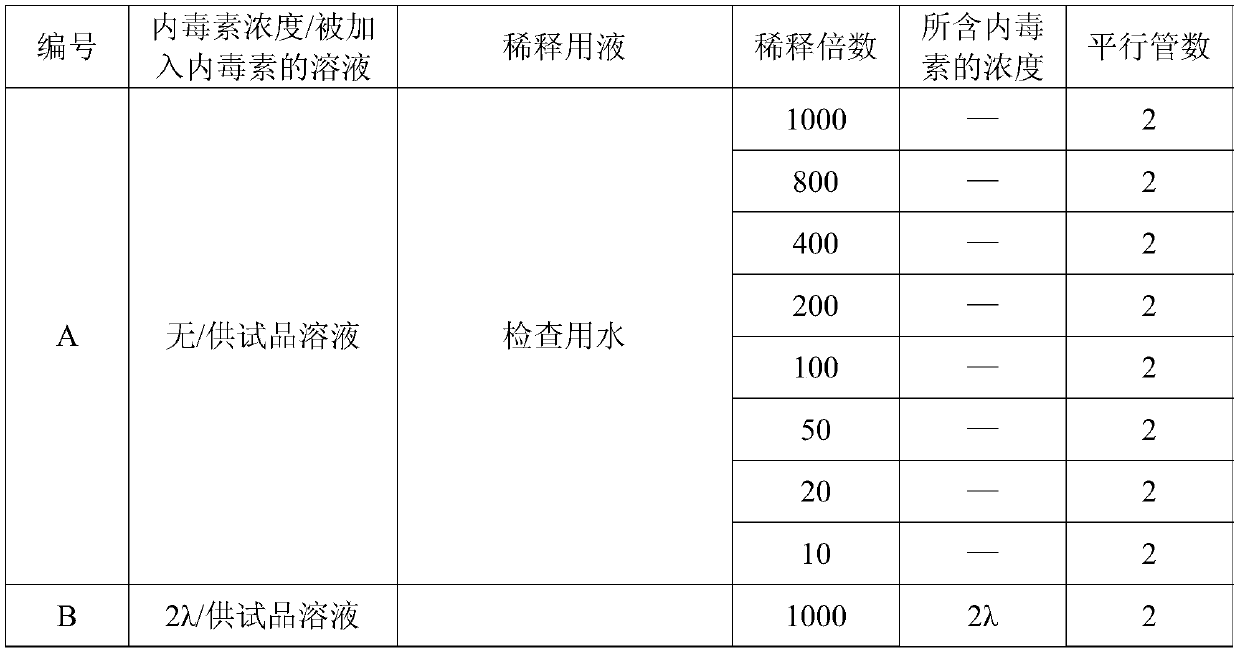
[0054] 3.1 Chloroform demulsification method Mix the tested IBD genetically engineered subunit oil emulsion vaccine with chloroform in equal amounts, place in a micro-vortex shaker for 6 minutes, then centrifuge at 8000rpm for 5 minutes, collect the precipitated The aqueous phase was tested for endotoxin. Prepare solutions A, B, C, and D according to Table 2, and use the gel limit test method for detection.
[0055] Table 3: Preparation table of gel semi-quantitative test solution
[0056]
[0057]
[0058] Judgment of results: When the dilution times of the positive control solution of the test product of B are 10 and 20, the result of the positive control solution of the test product is negative, indicating that the organic solvent has an impact on the detection of endotoxin, and the test is not established.
[0059] 3.2 Ultrasonic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com