A kind of content determination method of Xiebai Powder
A method of determination, the technology of Xiebai powder, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of not being able to fully and accurately reflect the quality of traditional Chinese medicine compositions, and the lack of content determination of various components in Xiebai powder, to achieve Good accuracy, strong specificity, and the effect of improving detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
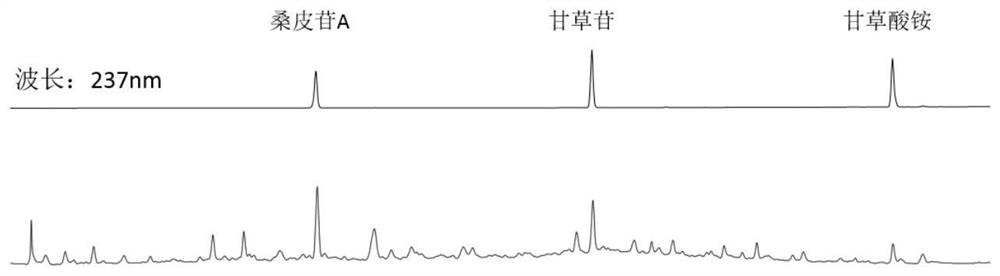
[0035] Adopt high-performance liquid chromatography to simultaneously measure the content of mulberry side A, liquiritin, and ammonium glycyrrhizin in Xiebai powder, and the operation method is as follows:
[0036] Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel was used as filler, 0.1% phosphoric acid solution was used as mobile phase A, acetonitrile was used as mobile phase B, and gradient elution was carried out according to Table 2; the flow rate was 1mL / min, the detection wavelength is 237nm, and the column temperature is 30°C.
[0037] Table 2 Gradient elution program
[0038]
[0039] Preparation of the reference substance solution: Accurately weigh 1 mg of the reference substances of molybdenum A, liquiritin, and ammonium glycyrrhizinate into a 10 mL volumetric flask, add 70% methanol solution to the volume to obtain a reference substance solution with a concentration of 0.1 mg / mL.
[0040] Preparation of the test solution...
Embodiment 2
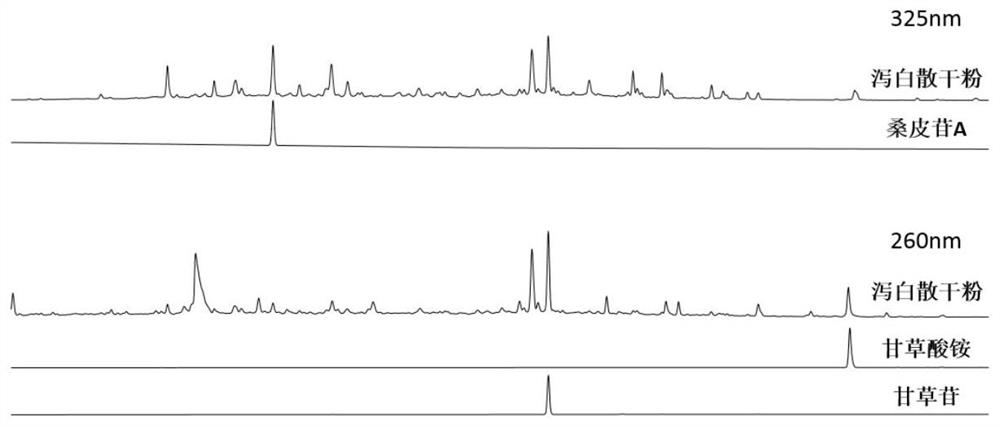
[0043] Adopt high-performance liquid chromatography to simultaneously measure the content of mulberry side A, liquiritin, and ammonium glycyrrhizin in Xiebai powder, and the operation method is as follows:
[0044] Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel was used as filler, 0.1% phosphoric acid solution was used as mobile phase A, acetonitrile was used as mobile phase B, and gradient elution was carried out according to Table 3; the flow rate was 1mL / min, the detection wavelength of molaritin A is 325nm, the detection wavelength of liquiritin and ammonium glycyrrhizinate is 260nm, and the column temperature is 30°C.
[0045] Table 3 Gradient elution program
[0046]
[0047] Preparation of the reference substance solution: Accurately weigh 1 mg of the reference substances of molybdenum A, liquiritin, and ammonium glycyrrhizinate into a 10 mL volumetric flask, add 70% methanol solution to the volume to obtain a reference sub...
Embodiment 3
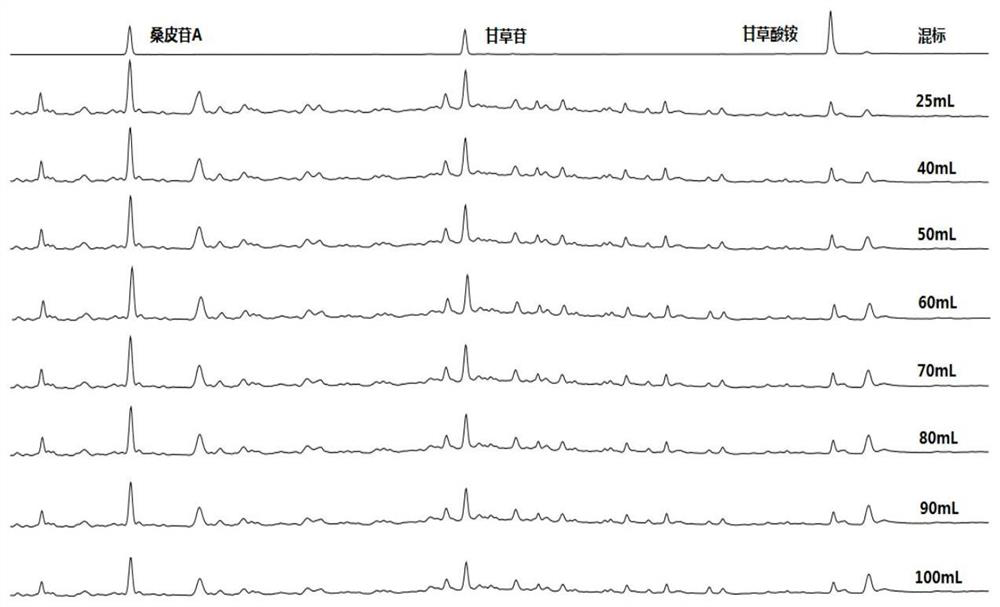
[0051] Selection of extraction solvent and its dosage
[0052] Chromatographic conditions: the organic phase is acetonitrile, the aqueous phase is 0.1% phosphoric acid solution, and the gradient elution is carried out according to Table 4; the flow rate is 1mL / min, the detection wavelength is 237nm, the column temperature is 30°C, and the injection volume is 5μL.
[0053] Table 4 Gradient elution program
[0054]
[0055]Plan 1: Take about 0.5g of Xiebai powder and spray dry powder, weigh it accurately, put it into a stoppered Erlenmeyer flask, add 70% ethanol 25mL, 40mL, 50mL, 60mL, 70mL, 80mL, 90mL, 100mL respectively, and seal it tightly. , weighed, sonicated for 30 minutes, allowed to cool, weighed again, supplemented the lost weight with 70% ethanol, shaken well, filtered, took 1 mL of the filtrate in a 1.5 mL EP tube, centrifuged for 10 minutes, and took the supernatant solution as the test solution. Carry out experiment according to above-mentioned chromatographic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com