A method for determining the fingerprint of compound Nanlangen granules
A technology of compound Nanbanlan and Nanlangen, which is applied in the field of traditional Chinese medicine, can solve problems such as the inability to control product quality as a whole, complex components of traditional Chinese medicine compound, and inability to fully reflect the overall quality characteristics of the product, achieving stable baseline, high precision, Good stability and reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Establishment of the Fingerprint Determination Method for Compound Nanlangen Granules
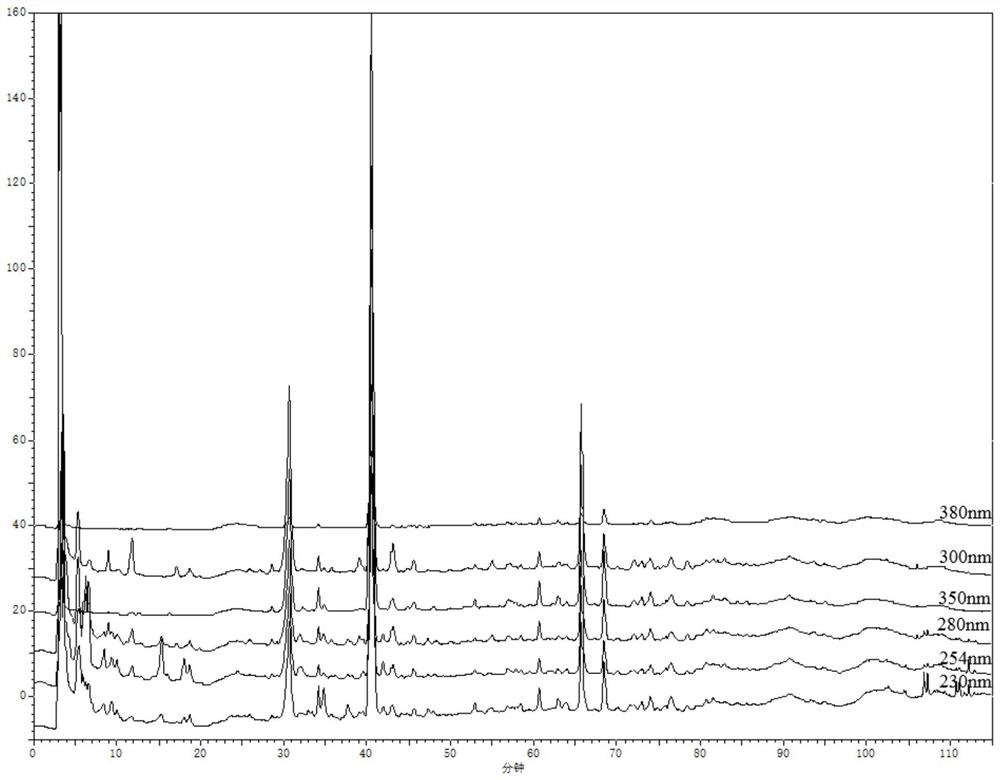
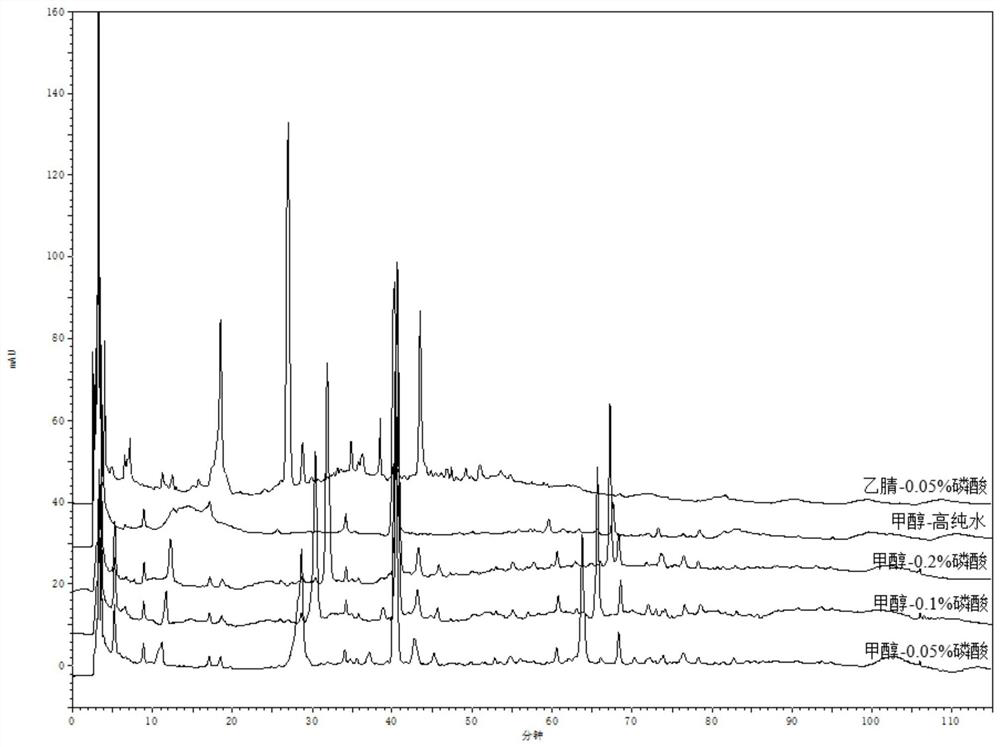
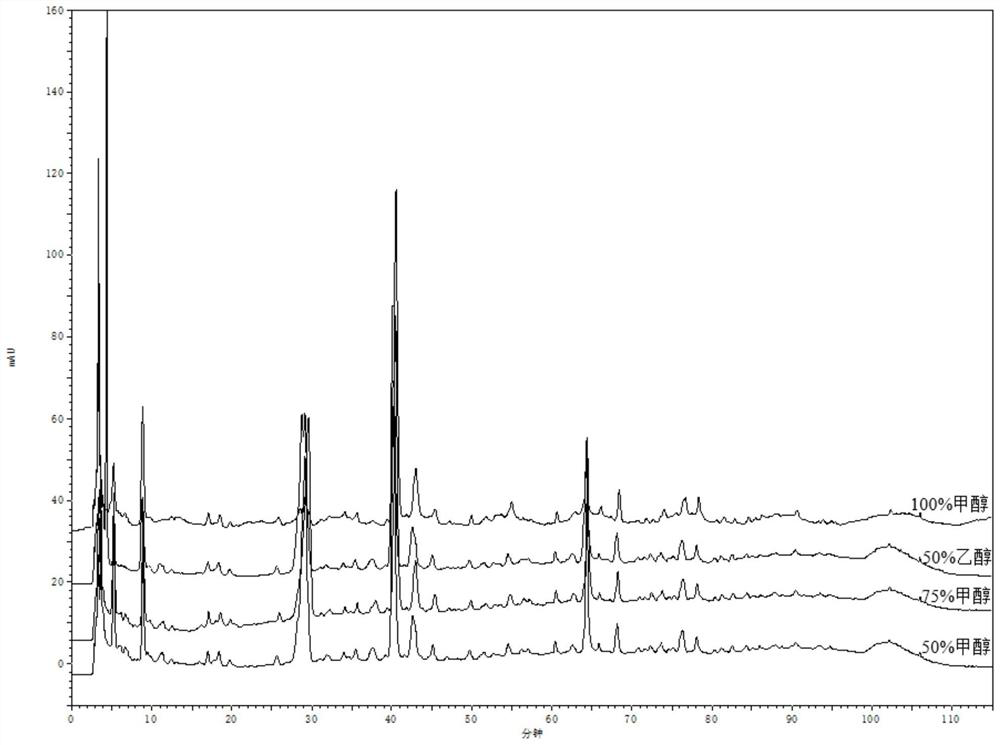
[0048] 1. Wavelength selection
[0049] Instrument: Agilent1260 high performance liquid chromatograph, ML204 / 02 analytical balance.
[0050] Reagents: HPLC analytical reagents are chromatographically pure, other reagents are analytically pure, water is ultrapure water, and compound Nanlangen granules are provided by Guangzhou Baiyunshan Qixing Pharmaceutical Co., Ltd.
[0051] Preparation of the test solution: accurately weigh about 10 g of compound Nanlangen granules, accurately add 25 mL of methanol solution with a volume concentration of 75% for extraction, weigh the weight, extract by ultrasonic (power 250W, frequency 50kHz) for 45min, return to room temperature and then extract Weigh the weight, use 75% methanol solution to supplement the lost weight, shake well, filter, filter with a 0.45 μm microporous membrane, and get the subsequent filtrate to obtain the test ...
Embodiment 2
[0074] Example 2: Quality detection method of compound Nanlangen granules based on fingerprint
[0075] Instrument: Agilent1260 high performance liquid chromatograph, ML204 / 02 analytical balance.
[0076] Reagents: acetaminophen B reference substance (batch number 200506), caffeic acid reference substance (batch number D11808026) were purchased from China National Institute for the Control of Pharmaceutical and Biological Products, the liquid chromatography analysis reagents were chromatographically pure, the remaining reagents were analytically pure, and the water was ultrapure water , Compound Nanlangen Granules are provided by Guangzhou Baiyunshan Qixing Pharmaceutical Co., Ltd.
[0077] Preparation of the test solution: Accurately weigh about 10g of compound Nanlangen granules, add 25mL of methanol solution with a volume concentration of 50% for extraction, weigh the weight, extract by ultrasonic for 45min, return to room temperature and weigh again, use 50% Methanol so...
Embodiment 3
[0083] Embodiment 3: methodological investigation
[0084] 3.1 Stability test
[0085] Taking the same batch of test samples (Guangzhou Baiyunshan Qixing Pharmaceutical Co., Ltd.), according to the best method for establishing fingerprints of compound Nanlangen granules, the fingerprints were detected at 0, 2, 4, 8, 12, and 24 hours respectively. With fencetin (peak No. 2) as the reference peak, record the chromatogram, calculate the relative retention time and relative peak area of each common peak chromatogram peak, the results are shown in Table 5 and Table 6, and the relative retention time RSD of each common peak is less than 1.0 %, the RSD of the relative peak area is less than 5.0%, indicating that the composition of the test solution is stable within 24h.
[0086] Table 5. Stability test-relative retention time ratio of main chromatographic peaks
[0087] Time / Peak ID 1 2 3 4 5 6 7 8 9 0h 0.715 1.000 1.063 1.122 1.500 1.590 1.692 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com