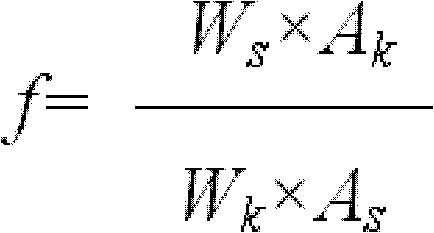Method for detecting tanshinone compounds in compound salvia tablets
A technology of compound danshen tablets and tanshinone, which is applied in the directions of measuring devices, instruments, scientific instruments, etc., can solve the problems of poor universality of the method, insufficient to fully reflect the quality of compound danshen tablets, and few monitoring indicators.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] According to the specification requirements of Compound Danshen Tablets in the 2010 edition of the Pharmacopoeia of the People's Republic of China, 20 batches of samples of Compound Danshen Tablets were taken for determination.
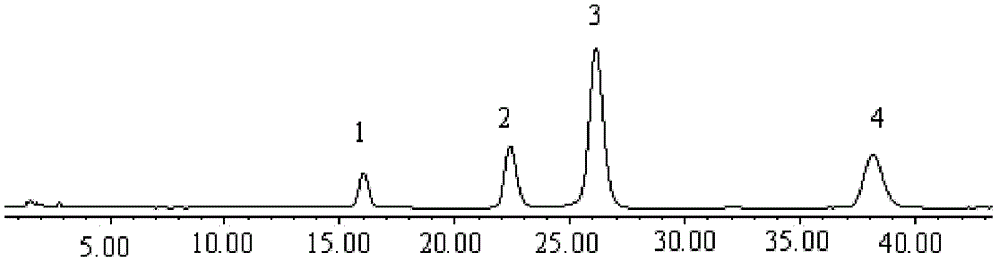
[0098] Determine according to high performance liquid chromatography (Appendix VI D of "Pharmacopoeia of the People's Republic of China" 2010 edition).
[0099] Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel is used as filler; methanol-water (73:27) is used as mobile phase; detection wavelength is 248nm. The number of theoretical plates according to Tanshinone II A The calculation should be no less than 2000.
[0100] Preparation of standard solution: take tanshinone compound reference substance, add methanol to dissolve it; in μg / mL, the mass volume ratio of dihydrotanshinone I reference substance to methanol is 10:1; cryptotanshinone reference substance, tanshinone I reference substance or Tanshino...
Embodiment 2
[0131] According to the specifications of Compound Danshen Tablets in the 2010 edition of the Pharmacopoeia of the People's Republic of China, 3 batches of Compound Danshen Tablets were taken for determination.
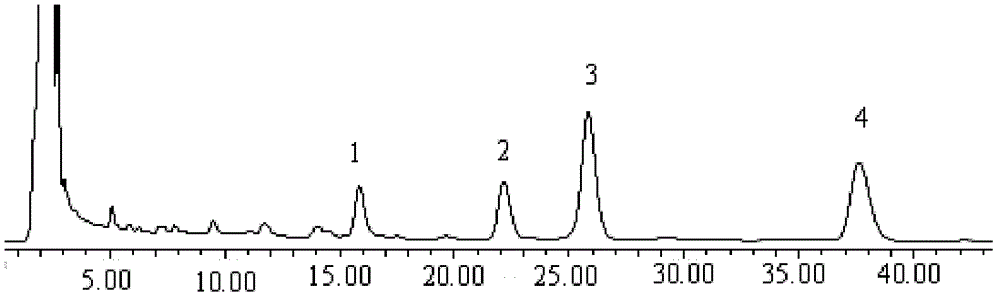
[0132] Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel is used as filler; methanol-water (70:30) is used as mobile phase; detection wavelength is 254nm. The number of theoretical plates according to Tanshinone II A The calculation should be no less than 2000.
[0133] Preparation of standard solution: Take tanshinone compound reference substance and dissolve it in methanol; in μg / mL, the mass volume ratio of dihydrotanshinone I reference substance to methanol is 8:1; cryptotanshinone reference substance, tanshinone I reference substance or Tanshinone II A The mass-to-volume ratio of the reference substance to methanol was 60:1.
[0134] Preparation of reference substance solution: take Tanshinone II A Take an appropriate a...
Embodiment 3
[0162] According to the specification requirements of Compound Danshen Tablets in the 2010 edition of the Pharmacopoeia of the People's Republic of China, 5 batches of Compound Danshen Tablets were taken for content determination.
[0163] Chromatographic conditions and system suitability test: octadecylsilane bonded silica gel is used as filler; methanol-water (75:25) is used as mobile phase; detection wavelength is 244nm. The number of theoretical plates according to Tanshinone II A The calculation should be no less than 2000.
[0164] Preparation of standard solution: take tanshinone compound reference substance, add methanol to dissolve it; in μg / mL, the mass volume ratio of dihydrotanshinone I reference substance to methanol is 12:1; cryptotanshinone reference substance, tanshinone I reference substance or Tanshinone II A The mass-to-volume ratio of the reference substance to methanol was 40:1.
[0165] Preparation of reference substance solution: take Tanshinone II A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com