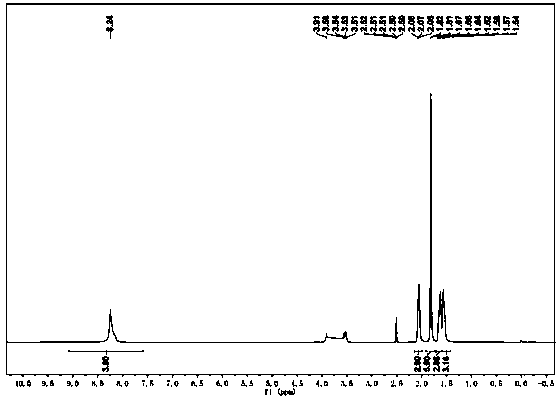
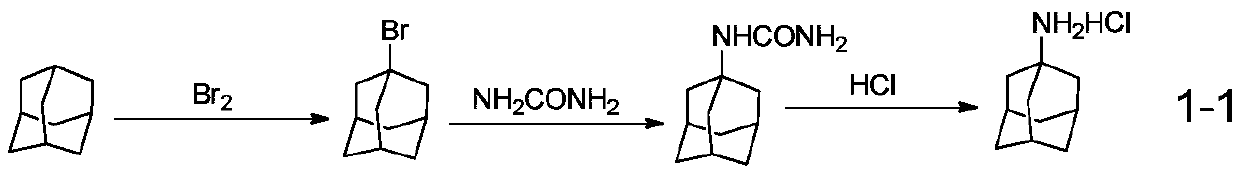
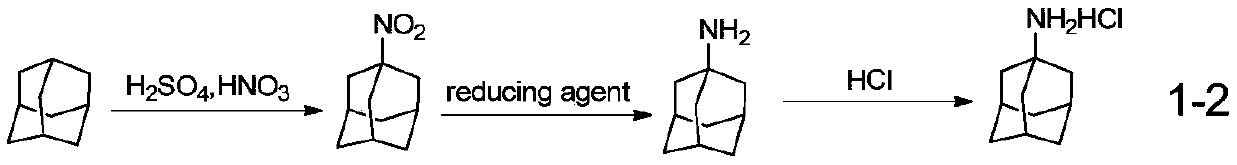
Synthetic method of amantadine hydrochloride
A technology for the synthesis of amantadine hydrochloride, which is applied in the preparation of carboxylic acid amides, chemical instruments and methods, and the preparation of amino compounds, etc., can solve problems such as excessive consumption, unrecyclable concentrated sulfuric acid and nitric acid, and rapid rise in reaction temperature. Achieve the effects of solving environmental pollution problems, good industrial application potential, and reducing waste gas production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] In order to solve the above problems, the first aspect of the present invention provides a kind of synthetic method of amantadine hydrochloride, comprising the following steps:
[0039] 1) Add adamantane, solvent I and initiator into the reaction kettle, and feed oxidant gas, and react at 30-100°C for 1-3 hours;
[0040] 2), add nitrile compound in step 1), react for 1~10h, after the reaction finishes, reclaim solvent I, obtain the solid A that sinks at the bottom of the kettle;
[0041] 3), the solid A obtained in step 2) is beaten and washed with the first mass part of water, and filtered to obtain acetylamantadine;
[0042] 4), put the acetylamantadine obtained in step 3) in an autoclave, dissolve it with solvent II, then add an alkaline substance and the second mass part of water, raise the temperature to 100-200°C, and react for 5-10 hours. , reclaim solvent II, and obtain solid B that sinks at the bottom of the kettle;
[0043] 5), adding the first mass part of ...
Embodiment 1
[0116] Embodiment 1 provides a kind of synthetic method of amantadine hydrochloride, comprising the following steps:
[0117] 1) Add 20g of adamantane, 120mL of solvent I and 0.16g of initiator into the reaction kettle, and feed oxidant gas, and react at 50°C for 2.5h;
[0118] 2), add 6.63g nitrile compound in step 1), react for 4h, after the reaction is finished, recover the solvent I, and obtain the solid A which sinks at the bottom of the kettle;
[0119] 3), the solid A obtained in step 2) was beaten and washed with 50 mL of water, and filtered to obtain acetylamantadine;
[0120] 4), put the acetylamantadine obtained in step 3) in an autoclave, add 35mL of solvent II to dissolve, then add 10.5g of alkaline substance and 5mL of water, heat up to 145°C, react for 8h, and recover the solvent after the reaction Ⅱ, obtain the solid B that sinks at the bottom of the kettle;
[0121] 5), add 50mL water to beat and wash solid B, filter to obtain solid C;
[0122] 6) Dissolve ...
Embodiment 2
[0146] Embodiment 2 provides a kind of synthetic method of amantadine hydrochloride, comprising the following steps:
[0147] 1) Add 20g of adamantane, 66mL of solvent I and 0.02g of initiator into the reaction kettle, and pass oxidant gas into it, and react for 2.5h at 50°C;
[0148] 2), add 2g nitrile compound in step 1), react for 4h, after the reaction finishes, recycle solvent I, obtain the solid A that sinks at the bottom of the kettle;
[0149] 3), the solid A obtained in step 2) was beaten and washed with 20 mL of water, and filtered to obtain acetylamantadine;
[0150] 4), put the acetylamantadine obtained in step 3) in an autoclave, add 26mL of solvent II to dissolve, then add 4g of alkaline substance and 2mL of water, raise the temperature to 145°C, and react for 8h. After the reaction, recover solvent II , to obtain the solid B sinking at the bottom of the kettle;
[0151] 5), add 20mL water to beat and wash solid B, filter to obtain solid C;
[0152] 6) Dissolv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com