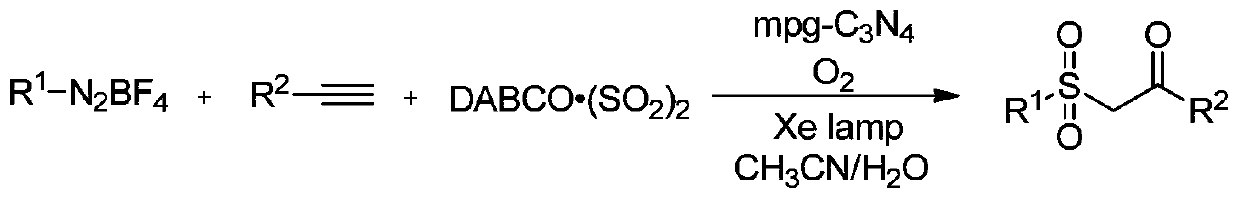Synthesis method of beta-ketosulfone compound
A synthesis method and compound technology, applied in the field of synthesis of β-ketosulfone compounds, can solve the problems of poor activity of terminal alkynes, high price, unfavorable production, etc., and achieve the effects of mild reaction conditions, high atom economy and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
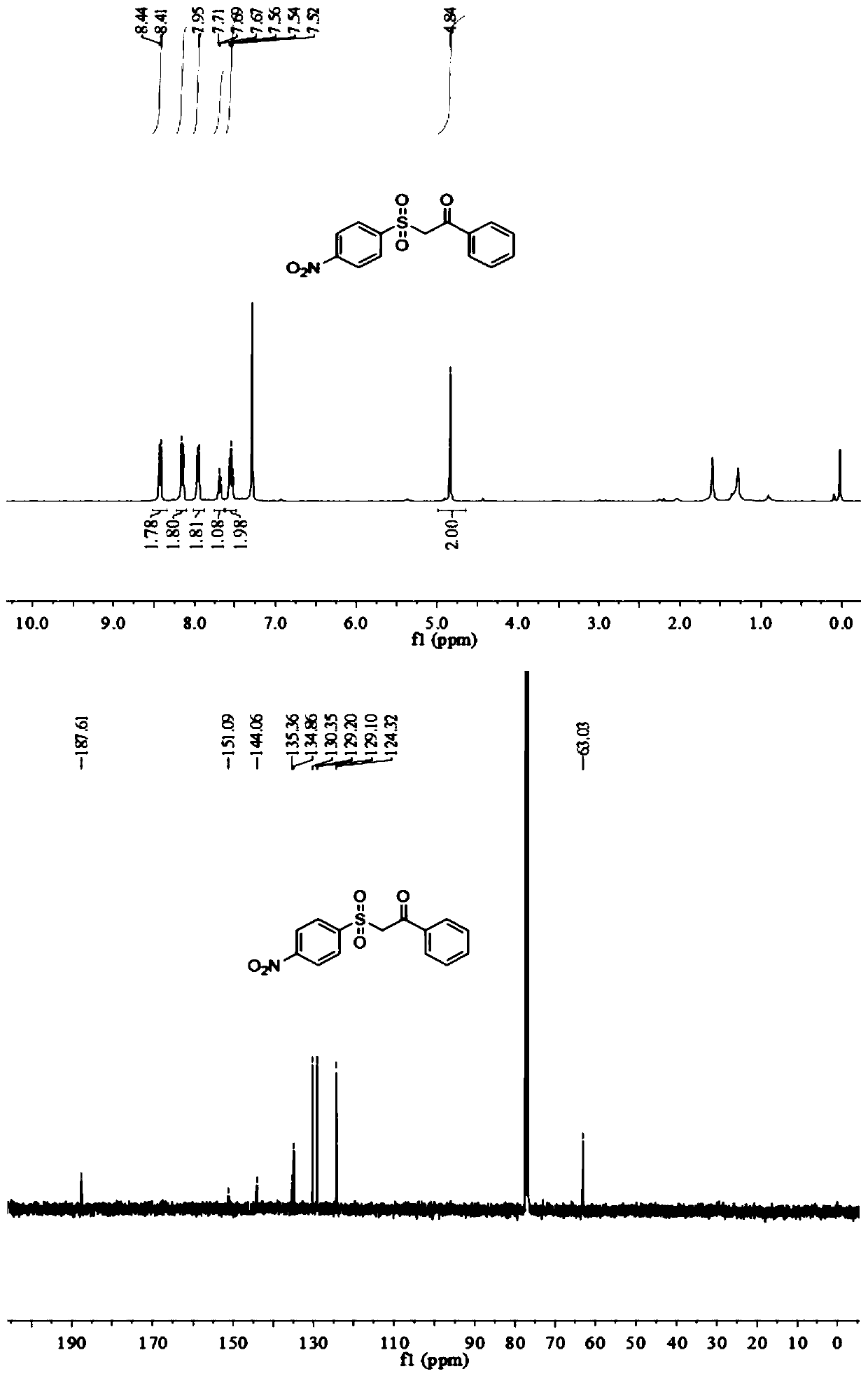
[0056] Add 0.5mmol p-nitroaryltetrafluoroborate diazonium salt, 0.6mmolDABCO·(SO 2 ) 2 , add 0.4mmol of phenylacetylene, add 2ml of acetonitrile aqueous solution, (acetonitrile: water = 1:1v / v), add 10mg of mesoporous carbon nitride, add a rotor, pour in oxygen, and stir magnetically at room temperature and under the irradiation of a 250-watt xenon lamp The device was stirred for 8h to end the reaction. The reaction product was separated and purified by column chromatography and thin layer chromatography using ethyl acetate and n-hexane as developing solvents (ethyl acetate:n-hexane=1:3), and the product was isolated. The calculated yield was 76%.
Embodiment 2
[0058] Add 0.5mmol p-methoxyaryltetrafluoroborate diazonium salt, 0.6mmolDABCO·(SO 2 ) 2 , add 0.4mmol phenylacetylene, add 2ml of acetonitrile solution (acetonitrile: water = 1:1), add rotor, add 10mg of mesoporous carbon nitride, add rotor, pour in oxygen, and stir magnetically at room temperature and under the irradiation of a 250-watt xenon lamp The device was stirred for 8h to end the reaction. The reaction product was separated and purified by column chromatography and thin layer chromatography using ethyl acetate and n-hexane as developing solvents (ethyl acetate:n-hexane=1:3), and the product was isolated. The calculated yield was 78%.
Embodiment 3
[0059] The influence of embodiment 3 visible light on synthesis
[0060] Add 0.5mmol p-nitroaryltetrafluoroborate diazonium salt, 0.6mmolDABCO (SO2) 2 in a 25ml pressure-resistant reaction tube, add rotor, add 0.4mmol phenylacetylene, add 2ml acetonitrile aqueous solution, (acetonitrile: water = 1:1 v / v), add 10 mg of mesoporous carbon nitride, add a rotor, pour in oxygen, stir with a magnetic stirrer at room temperature for 8 hours, no target product sulfone is formed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com