Alprostadil liposome and preparation method thereof
A technology of alprostadil and diltirrate, applied in the field of alprostadil liposome and its preparation, can solve the problems of inability to apply, the lungs are easily inactivated, affecting clinical effects, etc., so as to prolong the action time in vivo and solve the Harsh storage conditions and the effect of extended cycle times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
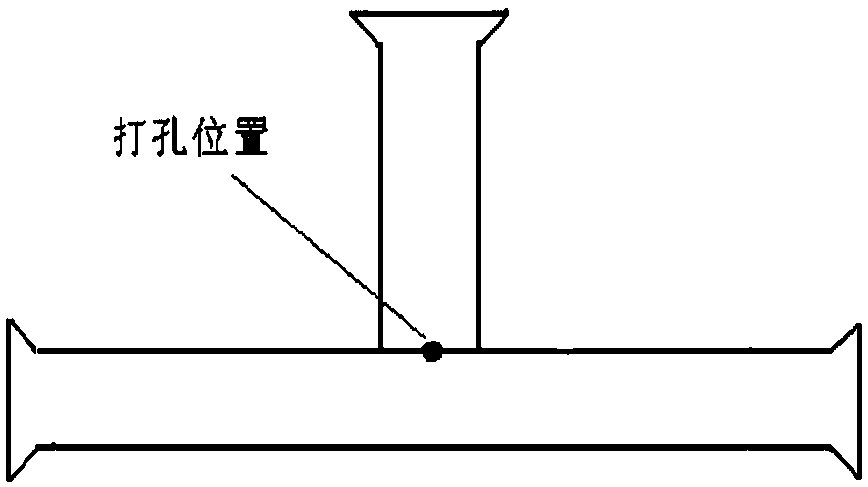
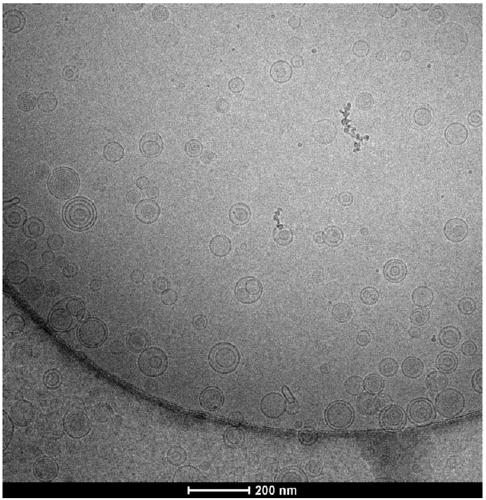
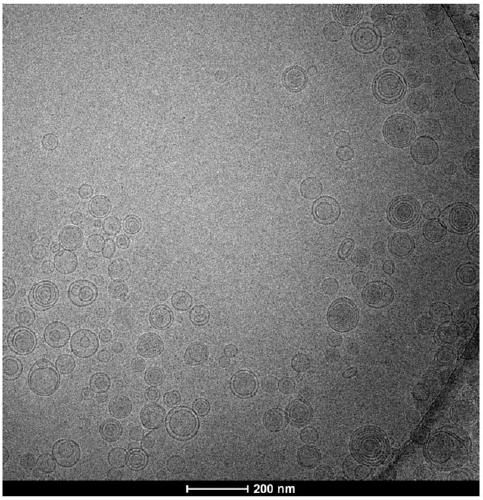
[0041] Embodiment 1: 300mg of alprostadil, 132g of egg yolk lecithin, 900mg of BHT are dissolved with 165ml of dehydrated alcohol to form a lipid phase solution, and 3000g of maltose is made into a water phase solution with a concentration of 10%, and the water phase solution is adjusted and the flow rate of the lipid phase solution are respectively 2000ml / min and 3ml / min, and then the ethanol solution is figure 1 Pour into the fast-flowing maltose solution. After the lipid phase solution is completely injected into the maltose solution, continue to stir for about 10 minutes to make the solution evenly mixed. The above-mentioned liposome solution is granulated with a high-pressure extruder, and a polycarbonate film of 0.45 μm + 0.22 μm + 0.1 μm is extruded 1-5 times, and the average particle size is controlled at 120-200nm. The diameter was detected by a Malvern laser particle size analyzer Zetasizer NanoZS, and the measured value was 147.4nm.
[0042] After the liquid medic...
Embodiment 2
[0045] Embodiment 2: 300mg of alprostadil, 90g of egg yolk lecithin, and 900mg of BHT are dissolved with 115ml of dehydrated alcohol to form a lipid phase solution, and 2400g of maltose is made into a water phase solution with a concentration of 8%, and the water phase solution is adjusted and the flow rate of the lipid phase solution are respectively 4000ml / min and 6ml / min, and then the ethanol solution is adopted figure 1 Pour into the fast-flowing maltose solution. After the lipid phase solution is completely injected into the maltose solution, continue to stir for about 20 minutes to make the solution evenly mixed. The above-mentioned liposome solution is granulated with a high-pressure extruder, and a polycarbonate film of 0.45 μm + 0.22 μm + 0.1 μm is extruded 1-5 times, and the average particle size is controlled at 120-200nm. The diameter detection adopts the Malvern laser particle size analyzer Zetasizer Nano ZS, and the measured value is 131.6nm, and the follow-up o...
Embodiment 3
[0046] Embodiment 3: the BHT of the alprostadil of 300mg, the egg yolk lecithin of 300g, the BHT of 1500mg are dissolved with the dehydrated alcohol of 375ml to form a lipid phase solution, and 3600g maltose is made into a water phase solution, and the concentration is 12% to adjust the water phase solution and The flow rate of lipid phase solution is 6500ml / min and 10ml / min respectively, and then the ethanol solution is adopted figure 1 Pour into the fast-flowing maltose solution. After the lipid phase solution is completely injected into the maltose solution, continue stirring for about 30 minutes to make the solution evenly mixed. The above-mentioned liposome solution is granulated with a high-pressure extruder, and a polycarbonate film of 0.45 μm + 0.22 μm + 0.1 μm is extruded 1-5 times, and the average particle size is controlled at 120-200nm. The diameter detection adopts the Malvern laser particle size analyzer Zetasizer Nano ZS, and the measured value is 174.1nm, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com