A kind of palladium catalyst carbon dioxide and the method for synthesizing α-acrylic acid compound of alkyne
A technology of carbon dioxide and compounds, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., to achieve the effects of mild conditions, wide substrate adaptability, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
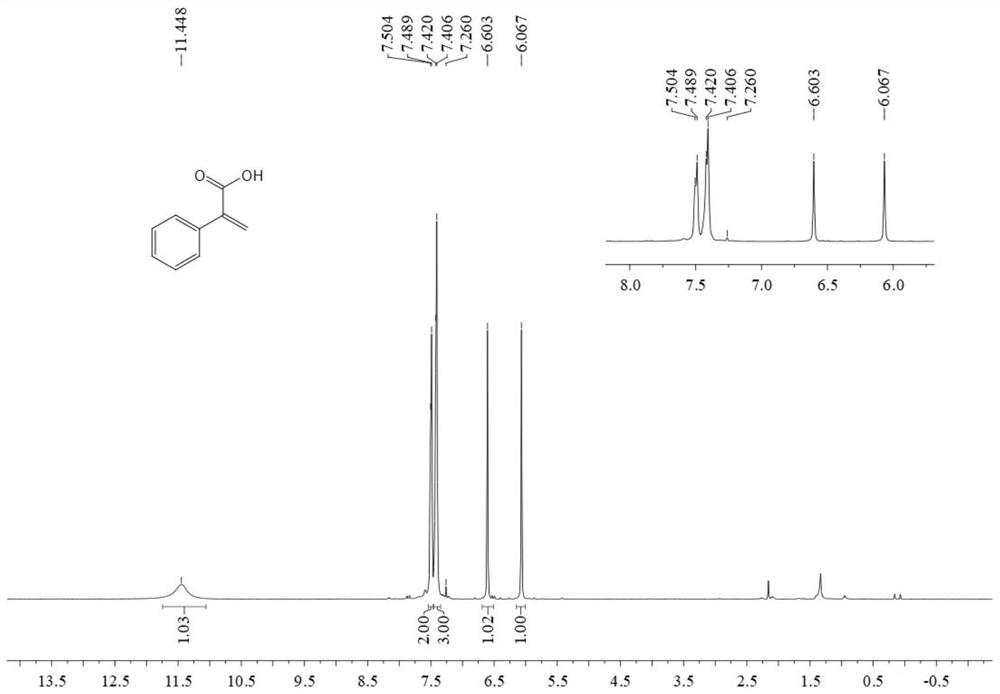
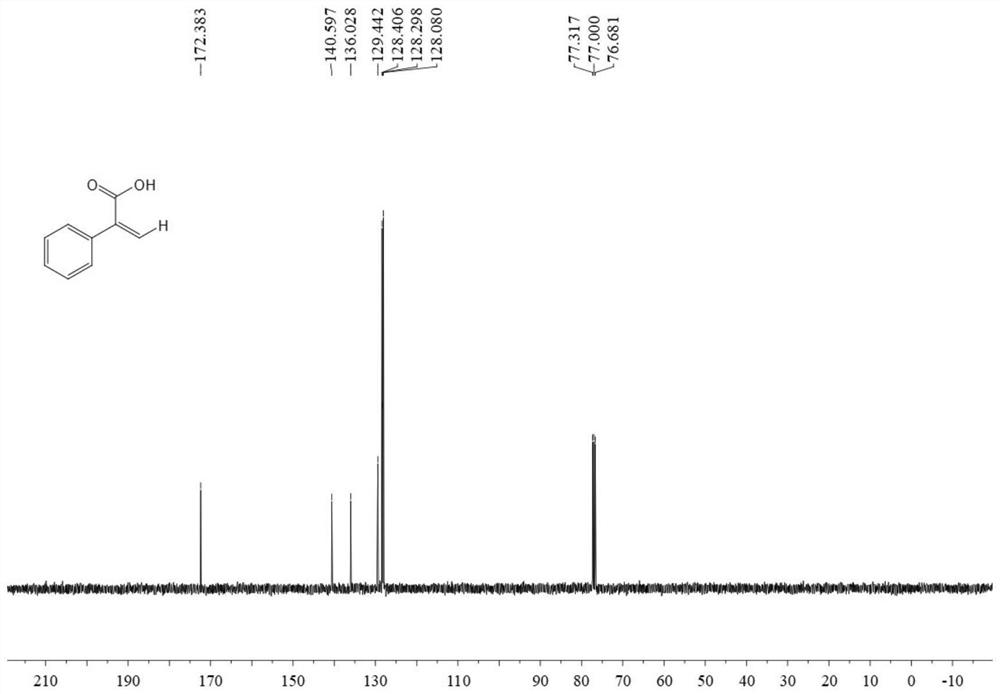
[0040] In the autoclave, sequentially add 0.6 mmol phenylacetylene, 0.009 mmol tetrakis(triphenylphosphine palladium), 0.009 mmol 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl , 0.6 mmoles of triethylamine, 0.78 mmoles of phenylsilane and 3 ml of N,N-dimethylformamide, into 2MPa carbon dioxide gas, stirred and reacted at 80°C for 12 hours, stopped heating and stirring, and cooled to room temperature , slowly venting unreacted CO 2 . The reaction solution was acidified with 3 ml of 2M hydrochloric acid, washed with water, extracted with ethyl acetate, the organic phases were combined, dried using anhydrous magnesium sulfate, filtered, and the solvent was removed by rotary evaporation under reduced pressure, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 2:1, and the yield was 82%.
[0041] The structural characterization data of the re...
Embodiment 2
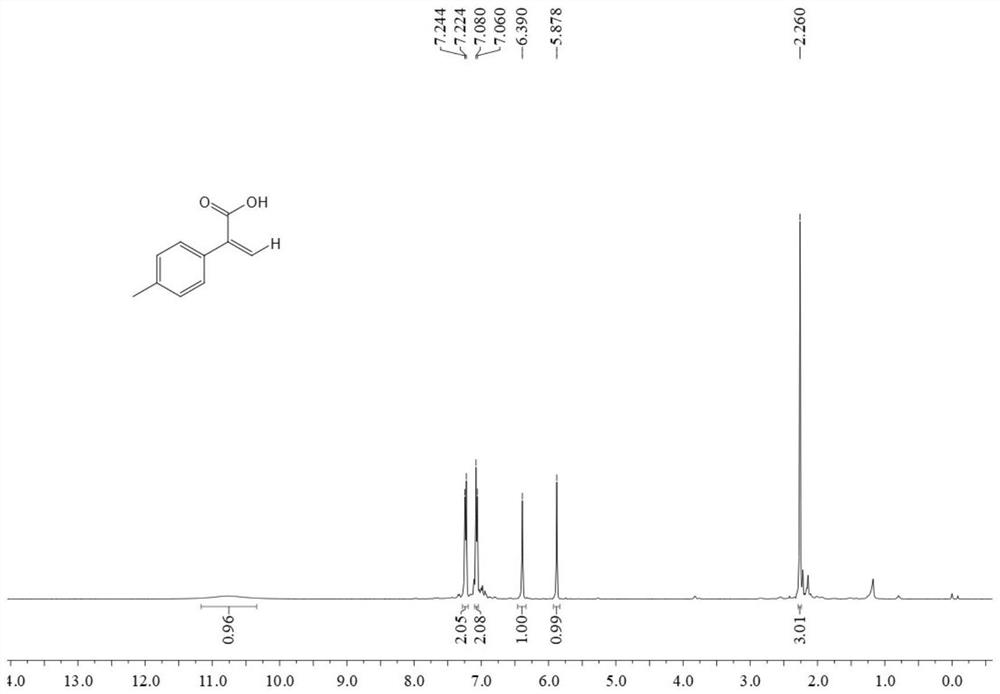
[0047] In the autoclave, sequentially add 0.6 mmol 4-methylphenylacetylene, 0.009 mmol tetrakis(triphenylphosphine palladium), 0.009 mmol 2,2'-bis-(diphenylphosphino)-1,1 '-binaphthylamine, 0.6 mmol triethylamine, 0.78 mmol phenylsilane and 3 ml N,N-dimethylformamide, 2 MPa carbon dioxide gas was passed through, and the reaction was stirred at 80°C for 12 hours, and the heating and stirring were stopped , cooled to room temperature, and slowly vented the unreacted CO 2 . The reaction solution was acidified with 3 ml of 2M hydrochloric acid, washed with water, extracted with ethyl acetate, the organic phases were combined, dried using anhydrous magnesium sulfate, filtered, and the solvent was removed by rotary evaporation under reduced pressure, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 2:1, and the yield was 79%.
[0048] The st...
Embodiment 3
[0054] In the autoclave, add 0.6 mmol 4-methoxyphenylacetylene, 0.009 mmol tetrakis(triphenylphosphine palladium), 0.009 mmol 2,2'-bis-(diphenylphosphino)-1, 1'-binaphthalene, 0.6 mmol triethylamine, 0.78 mmol phenylsilane, and 3 ml N,N-dimethylformamide were introduced into 2MPa carbon dioxide gas, stirred and reacted at 80°C for 12 hours, then stopped heating and Stir, cool to room temperature, and slowly vent unreacted CO 2 . The reaction solution was acidified with 3 ml of 2M hydrochloric acid, washed with water, extracted with ethyl acetate, the organic phases were combined, dried using anhydrous magnesium sulfate, filtered, and the solvent was removed by rotary evaporation under reduced pressure, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 2:1, and the yield was 83%.
[0055] The structural characterization data of the resul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com