Norovirus GI and GII type quantum dot joint inspection test strip as well as preparation method and application thereof
A technology of quantum dots and test strips, which is applied in the field of virus GI and GII quantum dot joint detection test strips and its preparation, can solve the problems of joint detection of norovirus GI and GII antigens that have not yet been seen, and achieve protection stability , high sensitivity, the effect of improving accuracy and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, a kind of norovirus GI and GII type antigen quantum dot joint detection test strip
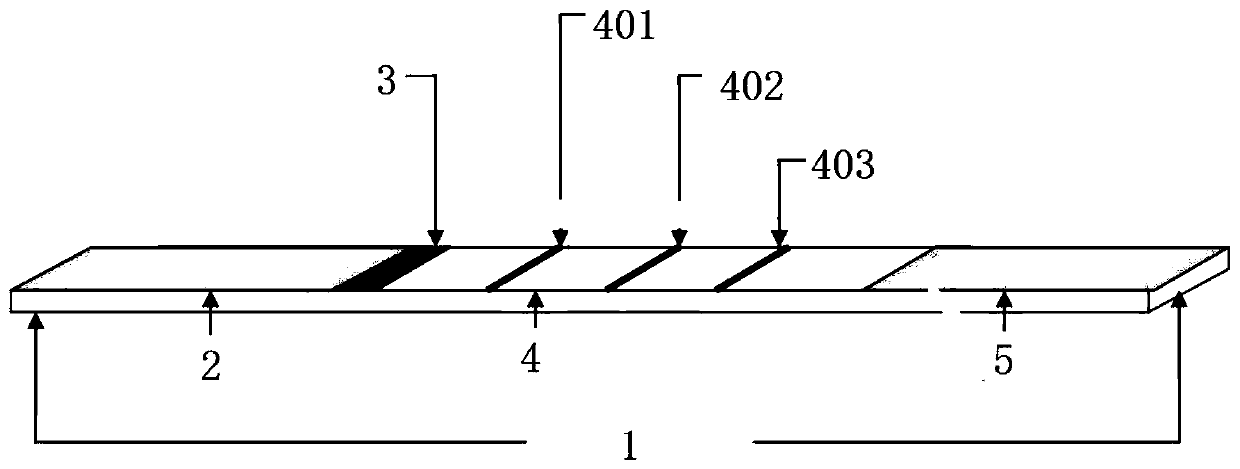
[0043] The test strip structure of this embodiment is as follows: figure 1 As shown, it includes a bottom plate 1, a sample pad 2, a binding pad 3, a nitrocellulose membrane 4, and a water-absorbing pad 5 overlapped successively on the bottom plate from left to right, and the binding pad is coated with quantum dot-labeled Nutraceuticals. Such as virus GI type detection antibody and quantum dot-labeled Norovirus GII type detection antibody; the nitrocellulose membrane is provided with 401 detection line 1, 402 detection line 2 and quality control line 403, and the detection line 1 is coated with There is norovirus type GI capture monoclonal antibody, and the detection line 2 is coated with norovirus type GII capture monoclonal antibody; the quality control line is coated with rabbit anti-mouse IgG polyclonal antibody.
[0044] Wherein, in this embodiment, the sample pad an...
Embodiment 2
[0046] Embodiment two, the preparation method of test strip of the present invention
[0047] (1) Preparation of sample pad
[0048] Cut the sample pad into an appropriate size and length that matches the binding pad, spray the sample pad treatment solution on the sample pad at an amount of 40 μL / cm, soak it at room temperature for 2 hours, and dry it in a 37°C thermostat for no less than 16 hours; Drying for use; the sample treatment solution is a phosphate buffer (0.01M, pH 7.2 ).
[0049] (2) Preparation of binding pads
[0050] ①Take 1 mg of carboxyl water-soluble quantum dots, and then suspend the quantum dots in 800 μL of MES buffer solution with a concentration of 0.05M and a pH of 6.0;
[0051] ②Use 0.05M pH6.0 MES buffer to prepare NHS (50mg / ml) and EDC (50mg / ml), take 20μl each and add to quantum dots, the final concentration is NHS (1mg / ml) and EDC (1mg / ml) ;
[0052] ③After activation for about 20 minutes, centrifuge and wash, 16000g at 4°C, 10min×2 times, dis...
Embodiment 3
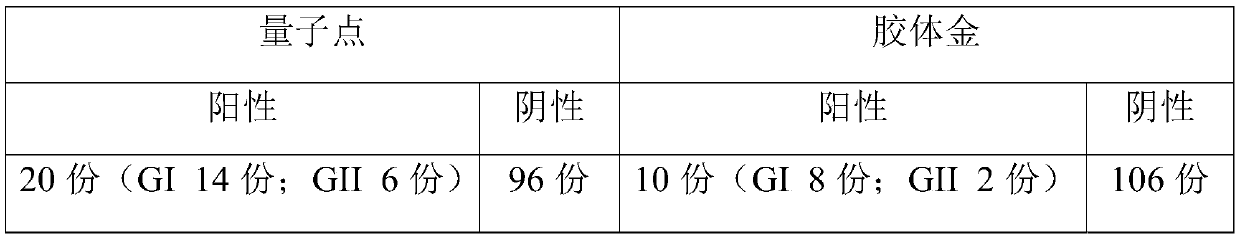
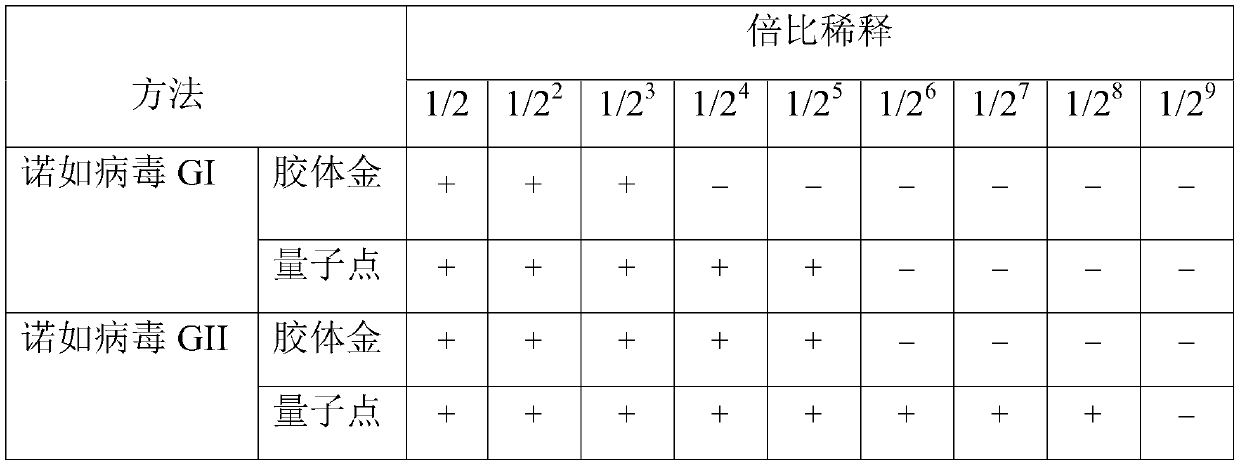
[0064] Embodiment 3, the screening experiment of quantum dot complex solution
[0065] Adopt different quantum dot complex solutions to resuspend quantum dot-labeled Norovirus GI type detection antibodies and quantum dot-labeled Norovirus GII type detection antibodies, process 1: quantum dot complex solutions contain 6 wt% of Turanose, Tween -40 0.08wt%, glycine 0.9wt% and ProClin950 0.06v% phosphate buffer (0.01M, pH 7.2); treatment 2: quantum dot complex solution containing Tween-40 0.08wt%, glycine 0.9wt% and ProClin950 0.06v% phosphate buffer (0.01M, pH 7.2); treatment: 3: Quantum dot recombination solution is phosphate buffer containing 6wt% turose, Tween-40 0.08wt% and ProClin950 0.06v% (0.01M, pH 7.2). The above-mentioned different quantum dot complex solutions were used to prepare each group of test strip samples according to the method described in Example 2, and each group of test strip samples prepared above was added dropwise to the negative control group for dete...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com