Triple inactivated vaccine and preparation method thereof
An inactivated vaccine and inactivated technology, applied in the field of vaccines, can solve problems such as loss of avian adenovirus breeding industry, and achieve the effects of good protection, low formaldehyde and endotoxin content, and strong immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The triple inactivated vaccine of the present embodiment, the antigen of the triple inactivated vaccine is the inactivated Newcastle disease La Sota virus strain, the inactivated H9 subtype avian influenza QF01 strain and the inactivated I group 8b avian adenovirus Hexon protein The amino acid sequence of the I group 8b type avian adenovirus Hexon protein is shown in SEQ ID NO.2; the nucleotide of the I group 8b type avian adenovirus Hexon protein is shown in SEQ ID NO.1; Chicken Newcastle disease La Sota virus strain content ≥ 10 8.5 EID 50 / 0.1mL; the content of the H9 subtype avian influenza QF01 strain ≥ 10 7.5 EID 50 / 0.1mL; the agar amplification titer of described I group 8b type avian adenovirus Hexon protein content ≥ 1:32;
[0040] The chicken Newcastle disease La Sota virus strain is derived from China Veterinary Medicine Supervision Institute;
[0041] The H9 subtype avian influenza QF01 strain belongs to influenza virus and was preserved in the General ...
Embodiment 2
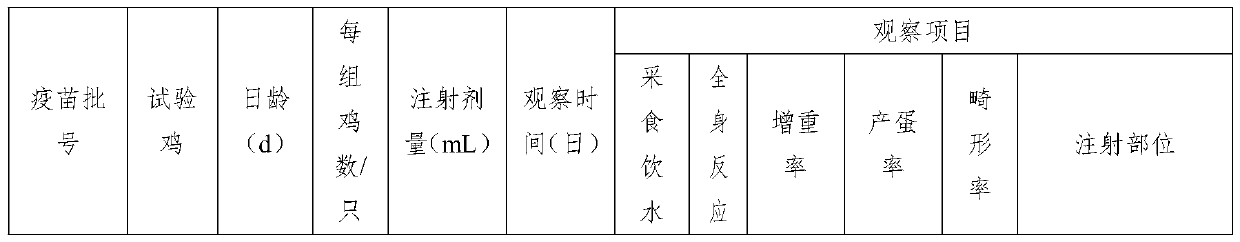
[0078] Prepare the triple inactivated vaccine of three batches according to the preparation method of the triple inactivated vaccine of embodiment 1, batch number is respectively NAFs01, NAFs02 and NAFs03, carry out safety test respectively:
[0079] (1) Single-dose one-time vaccination safety test
[0080] Get 10 days old SPF (no specific pathogen) chicken, the injection dosage of described triple inactivated vaccine is 0.3mL / , 10 / group, totally three groups, the 10 days old SPF chicken injection batch number of the first group is NAFs01 Triple inactivated vaccine, the 10-day-old SPF chicken injection batch number of the second group is the triple inactivated vaccine of NAFs02, and the 10-day-old SPF chicken injection batch number of the third group is the triple inactivated vaccine of NAFs03; The 10-day-old SPF chicken of the inactivated vaccine is a negative control group, and the injection dose of normal saline injected into the neck of the 10-day-old SPF chicken of the ne...
Embodiment 3
[0116] Be respectively the triple inactivated vaccine of three batches of NAFs01, NAFs02 and NAFs03 with the batch number among the embodiment 2, carry out immune efficacy test respectively:
[0117] (1) Efficacy experiment of chicken Newcastle disease virus
[0118] The efficiency experiment of chicken Newcastle disease virus was carried out by serological method and immune challenge method respectively;
[0119] (1) Serological method: get 21 day old SPF chickens, the immunization dosage of the subcutaneous inoculation of described triple inactivated vaccine is 20 μ L / , 10 / group, altogether three groups, described three groups are immunization groups, the first One group of 21-day-old SPF chickens was subcutaneously inoculated with the triple inactivated vaccine with batch number NAFs01; The triple inactivated vaccine of NAFs03; Establish simultaneously 5 21-day-old SPF chickens without injecting triple-inactivated vaccines as negative control group, and the 21-day-old SPF ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com