Linker, drug-loaded linker, cell-penetrating peptide-coupled drug, antibody-coupled drug and preparation method thereof
A linker and drug-loading technology, applied to the linker, can solve the problems of toxic and side effects, and achieve the effect of controllable delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Take by weighing the compound shown in the formula (a1) of 1.0eq (wherein, in formula (a1), " R 3 ” is benzoyl), dissolved with 60mL of anhydrous dimethylformamide under the protection of argon; then added 3.0eq imidazole and 1.5eq tert-butyldiphenylchlorosilane to the reaction system. Diluted with ethyl ester, washed with water, dried the separated organic phase with anhydrous magnesium sulfate, filtered, concentrated, and sucked dry to obtain the compound represented by the crude product formula (a2).
[0126] Get last step product (wherein, in formula (a2), " R 2 "is tert-butyldiphenylsilyl) dissolved in 60mL of dichloromethane, added 7.0mL of 25% by mass sodium methoxide methanol solution, stirred at room temperature for 1h. Concentrated under reduced pressure, separated by column chromatography to obtain two isomers , 85% yield in two steps.
[0127] Isomer a: 1 H NMR (400MHz, CDCl 3 )δ7.65-7.62(m,4H),7.44-7.38(m,6H),5.12(dd,J=5.6,2.0Hz,1H),4.51-4.47(m,1H),4.10...
Embodiment 2
[0148] This example is to illustrate the process of preparing a cell-penetrating peptide-conjugated drug by coupling the drug-loaded linker prepared in Example 1 with a cell-penetrating peptide.
[0149] Include the following steps:
[0150] 1) The drug-loaded linker Mal-PC4AP-DOX prepared in Example 1 was dissolved in dimethylformamide to obtain a stock solution of the drug-loaded linker with a concentration of 1.5 mM. The H3-V45C protein was dissolved in sterile water to obtain a stock solution with a concentration of 300 μM.
[0151] 2) Mix 33.5 microliters of drug-loaded linker stock solution with 160 microliters of H3-V35C protein in HEPES buffer at pH 7.5 (20 mM), and incubate at 37° C. for 1 hour.
[0152] 3) After the reaction, the obtained cell-penetrating peptide-conjugated drug (H3-PC4AP-DOX, whose structure is shown in Formula 5) needs to be further purified and desalted using PD MiniTrap G-25 desalting column. Specific desalting method: load 200 microliters of s...
Embodiment 3
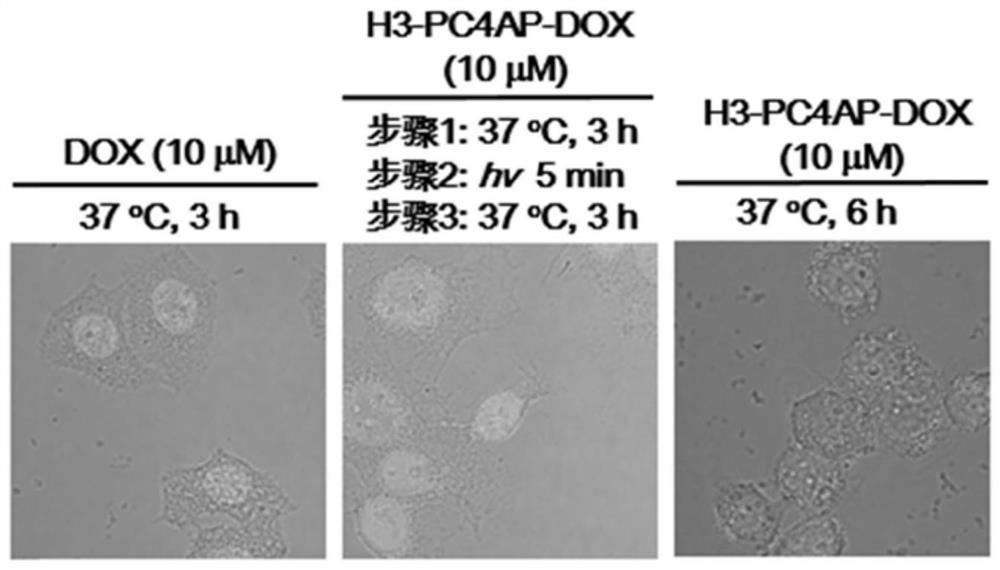
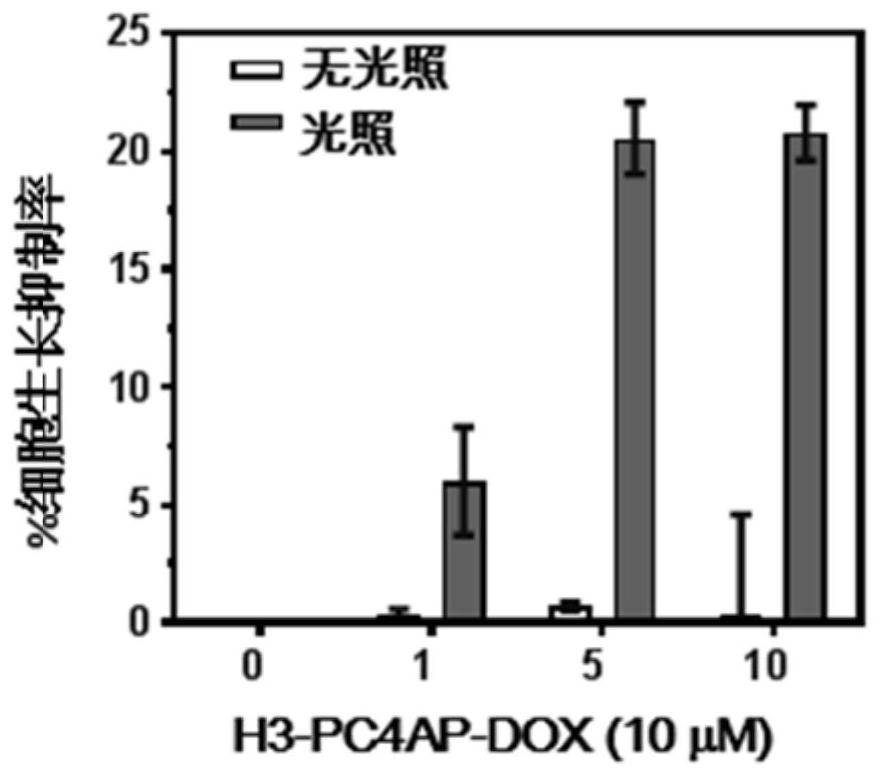
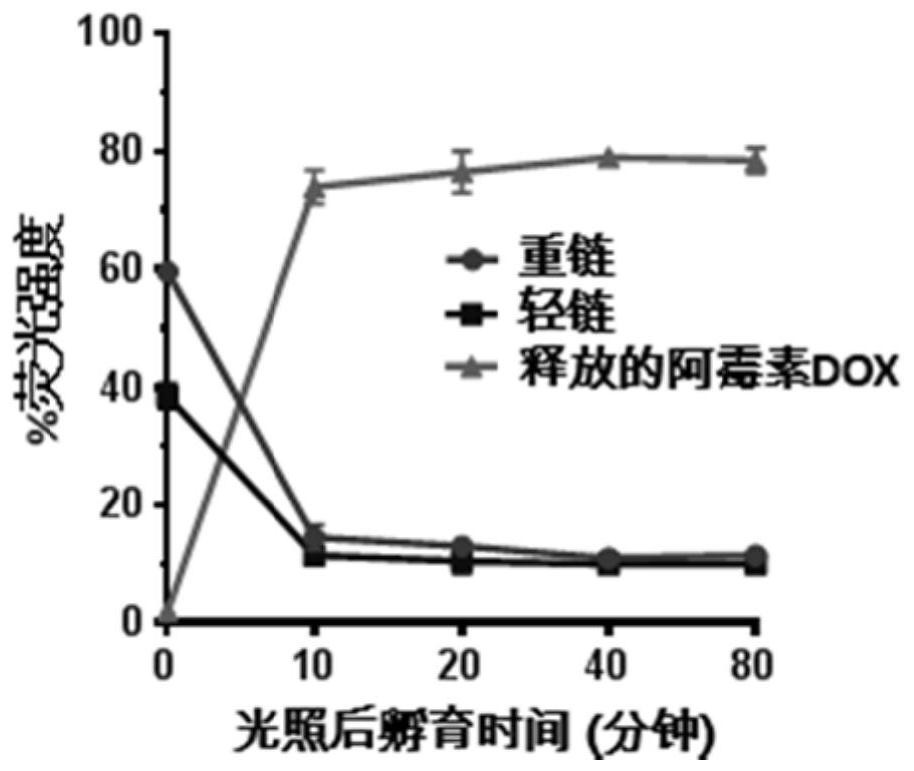
[0154] This example is to illustrate that the cell-penetrating peptide-conjugated drug H3-PC4AP-DOX prepared in Example 2 can be used for light-controlled release of doxorubicin DOX in HeLa cells.
[0155] 3.1 Confocal microscopy to detect the cellular localization of DOX and H3-PC4AP-DOX
[0156] HeLa cells in logarithmic growth phase were digested with trypsin, washed and centrifuged to prepare single cell suspension. The counted cells were seeded in a 24-well plate (density 1.0×10 5 per well) at 37°C containing 5% CO 2 overnight in a constant temperature incubator. After overnight culture, the cells were washed twice with PBS, and the complete medium containing 10 μM doxorubicin and H3-PC4AP-DOX were added respectively. After continuing to culture for different periods of time, the original culture solution was sucked off, the cells were soaked twice in PBS, and fixed with 300 μL of 4% paraformaldehyde at room temperature for 20 minutes. After soaking in PBS for 2 times...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com