Sodium ferulate ophthalmic preparation and preparation method thereof
A technology of sodium ferulate and ophthalmic preparations, which is applied in the field of sodium ferulate ophthalmic preparations and its preparation, can solve the problems of complicated Chinese medicine ingredients, inaccurate curative effects, and difficult effective inspection, and achieve single ingredients, good performance, High practical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The eye drops of this embodiment include active ingredients, osmotic pressure protectors, osmotic pressure regulators, pH regulators, viscous agents and bacteriostatic agents, and the solvent is water for injection; see Table 1.
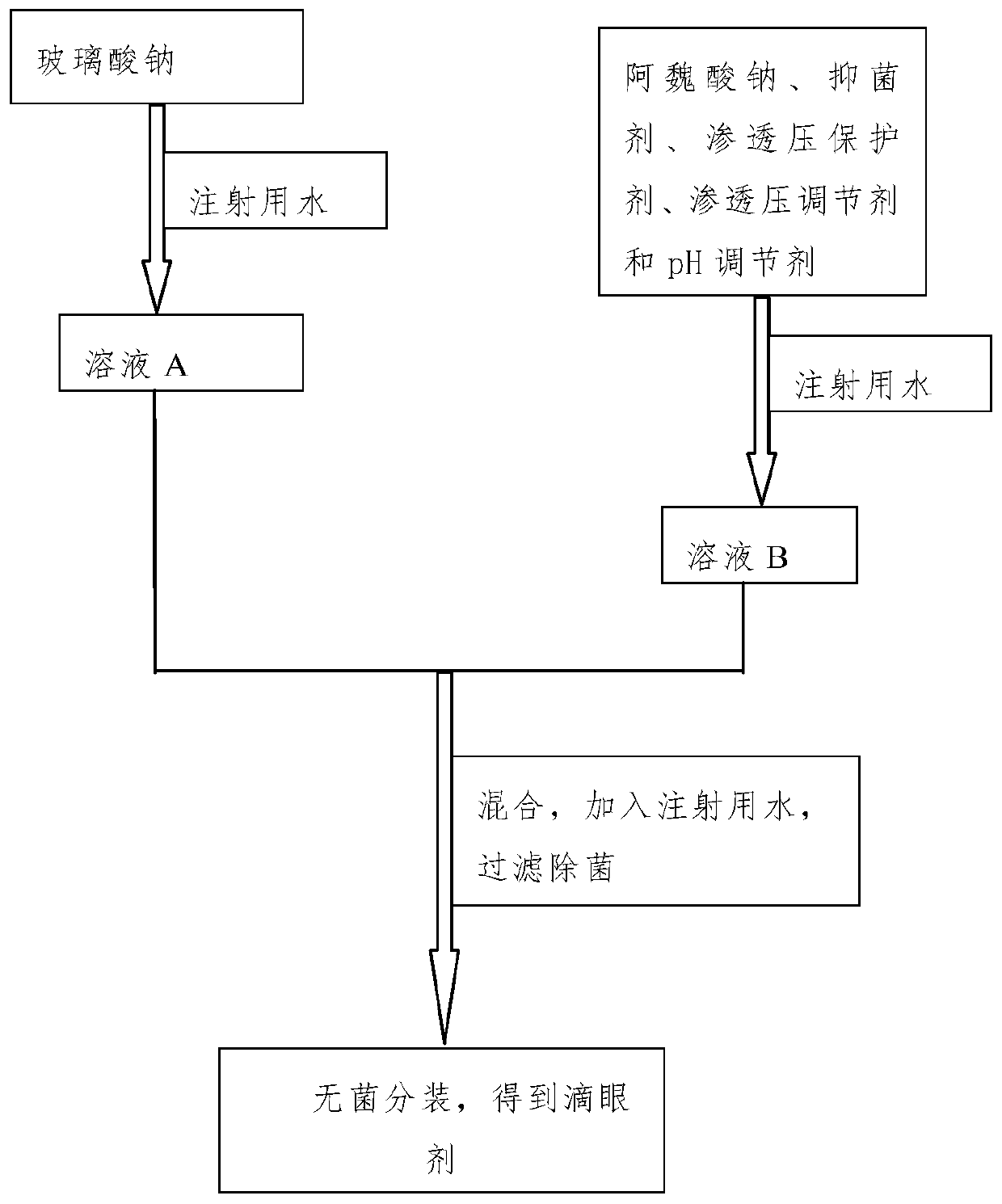
[0041] The eye drops of this embodiment are prepared under aseptic conditions, such as figure 1 Shown, the preparation method is:
[0042] Step 1, dissolving the viscous agent sodium hyaluronate in water for injection, and obtaining solution A after refrigeration;
[0043] Step 2, dissolve the bacteriostatic agent ethyl p-hydroxybenzoate in water for injection under heating conditions, stir, then cool, and add active ingredient sodium ferulate, osmotic pressure protecting agent taurine, The osmotic pressure regulator sodium chloride and the pH regulator sodium bicarbonate obtain solution B;
[0044] Step 3: Mix the solution A described in step 1 with the solution B described in step 2, then add water for injection to 1000 mL, stir evenly, f...
Embodiment 2
[0047] This example is the same as Example 1, except that the osmoprotectant is trehalose, carnitine or betaine, or two of trehalose, taurine, carnitine and betaine. The osmotic pressure regulator is glucose; the pH regulator is sodium hydroxide, sodium dihydrogen phosphate, disodium hydrogen phosphate, citric acid, sodium citrate, boric acid or borax, or sodium bicarbonate , sodium hydroxide, sodium dihydrogen phosphate, disodium hydrogen phosphate, citric acid, sodium citrate, boric acid and borax; the antibacterial agent is propyl p-hydroxybenzoate, p-hydroxybenzoic acid methyl esters, benzalkonium chloride, benzalkonium bromide, chlorobutanol, thimerosal, chlorhexidine, benzyl alcohol, or benzoic acid;
[0048] The preparation method of the eye drops of this embodiment is the same as that of Example 1.
[0049] The eye drops in this example have an osmotic pressure of 280mOsm / L-290mOsm / L, and a pH value of 6.5-7.5.
Embodiment 3
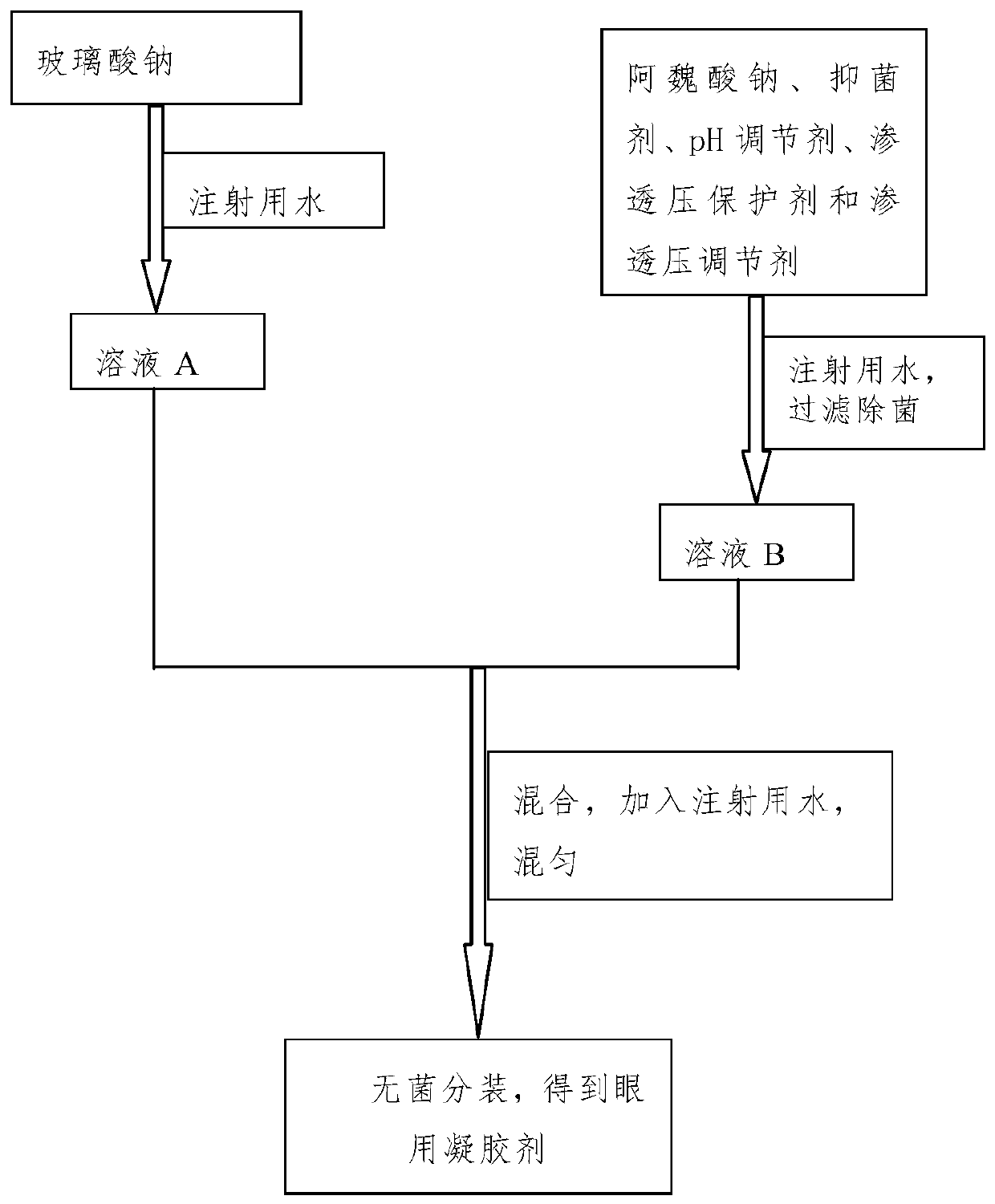
[0051] The eye drops of this embodiment include active ingredients, osmotic pressure protectors, osmotic pressure regulators and viscous agents, and the solvent is water for injection; the types and contents of raw materials contained in each 1000mL eye drops are shown in Table 1.
[0052] The eye drops of the present embodiment are prepared under aseptic conditions, and the preparation method is:
[0053] Step 1, dissolving the viscous agent sodium hyaluronate in water for injection, and obtaining solution A after refrigeration;
[0054] Step 2, adding the active ingredient sodium ferulate, the osmotic pressure protecting agent trehalose and taurine, and the osmotic pressure regulator glucose to the water for injection to obtain solution B;
[0055] Step 3: Mix the solution A described in step 1 with the solution B described in step 2, then add water for injection to 1000 mL, stir evenly, filter and sterilize through a microporous membrane, and pack aseptically to obtain eye ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com