Acid catalytic synthesis method and application of phenoxyacetate derivatives
A technology of phenoxy acetate and synthesis method, which is applied in the direction of chemical instruments and methods, drug combination, carboxylate preparation, etc., can solve the problems of heavy metal ions being greatly affected and not conforming to environmental protection, etc., so as to promote the occurrence of reactions, It has the effect of structural diversity and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
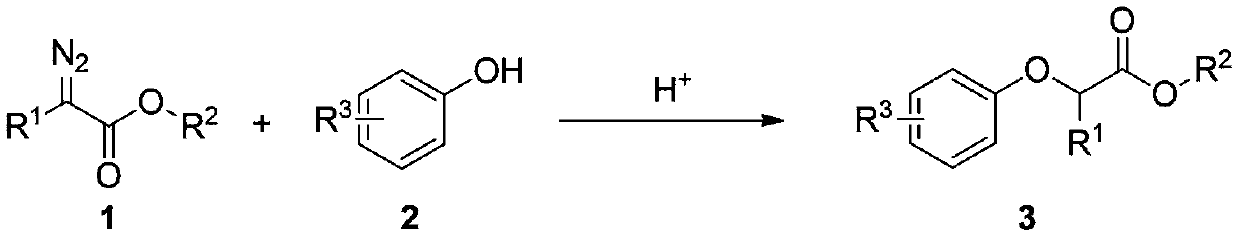
[0040] At room temperature, successively weigh ethyl phenyldiazoacetate 1a (0.5mmol) and phenol 2a (0.8mmol) into a 25mL Schlenk reaction tube, add 5mL of 1,2-dichloroethane under air atmosphere, Stir at room temperature for 2 minutes, slowly add 0.1 mmol (10 μL) of 98% trifluoromethanesulfonic acid into the micro-injector, continue the reaction at room temperature for 20 minutes, TLC detects that the raw materials have reacted completely, stop the reaction; concentrate under reduced pressure to remove volatile components, Silica gel column chromatography (eluent: petroleum ether (60-90°C) / ethyl acetate, v / v=10:1) gave the target product 3a (83 mg, yield 65%) in a colorless, transparent and viscous form , the target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 2
[0041] Embodiment 2 (comparative example: different catalyst contrasts)
[0042]
[0043] At room temperature, successively weigh ethyl phenyldiazoacetate 1a (0.5mmol) and phenol 2a (0.8mmol) into a 25mL Schlenk reaction tube, add 5mL of 1,2-dichloroethane under air atmosphere, Stir at room temperature for 2 minutes, slowly add 96% sulfuric acid 0.1mmol (5.3μL) into the micro-injector, continue to react at room temperature for 20min, TLC detects that the raw materials have reacted completely, stop the reaction; concentrate under reduced pressure to remove volatile components, silica gel column chromatography Separation (eluent: petroleum ether (60-90°C) / ethyl acetate, v / v=10:1), the target product 3a (58 mg, yield 45%) was obtained as a colorless, transparent and viscous product, the target product Confirmed by NMR and high-resolution mass spectrometry.
Embodiment 3
[0044] Embodiment 3 (comparative example: different catalyst contrasts)
[0045]
[0046] At room temperature, successively weigh ethyl phenyldiazoacetate 1a (0.5mmol) and phenol 2a (0.8mmol) into a 25mL Schlenk reaction tube, add 5mL of 1,2-dichloroethane under air atmosphere, Stir at room temperature for 2 minutes, slowly add 72% perchloric acid 0.1mmol (7.9μL) into the micro-injector, continue to react at room temperature for 20min, TLC detects that the raw materials have reacted completely, stop the reaction; concentrate under reduced pressure to remove volatile components, silica gel Column chromatography (eluent: petroleum ether (60-90°C) / ethyl acetate, v / v=10:1) gave the target product 3a (78 mg, yield 55%) in a colorless, transparent and viscous form. The target product was confirmed by NMR and high-resolution mass spectrometry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com