Recombinant baculovirus, raccoon dog parvovirus recombinant subunit vaccine and preparation method of raccoon dog parvovirus recombinant subunit vaccine
A technology for recombining baculovirus and parvovirus, applied in the fields of botanical equipment and methods, biochemical equipment and methods, vaccines, etc., can solve the problems of high serum prices, virus destruction, incomplete inactivation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0072] The preparation method of the raccoon parvovirus recombinant subunit vaccine of the present invention is a full-suspension, serum-free culture method, which mainly includes the following steps:
[0073] Step 1. Prepare seed cells by culturing high Five cells in serum-free full suspension in Erlenmeyer shake flasks;
[0074] Step 2. Inoculate the seed cells directly into the bioreactor to culture high Five cells in full suspension, and enlarge the cultivation scale of the bioreactor to a 30L cell tank;
[0075] Step 3: Culture the recombinant baculovirus in full suspension without serum in a conical flask, inoculate it into the cultured cells in the above 30L bioreactor at a multiplicity of infection of 0.5-5.0, and culture for 72-140 hours;
[0076] Step 4: Harvest the supernatant of the above-mentioned culture solution, inactivate it, add aluminum hydroxide glue and mix it to make a vaccine, that is, the raccoon parvovirus recombinant subunit vaccine.
[0077] The rac...
Embodiment 1
[0079] The construction of embodiment 1 recombinant baculovirus
[0080] 1. Acquisition of raccoon parvovirus (RDPV) structural protein VP2 gene
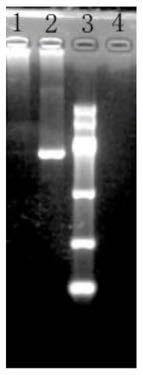
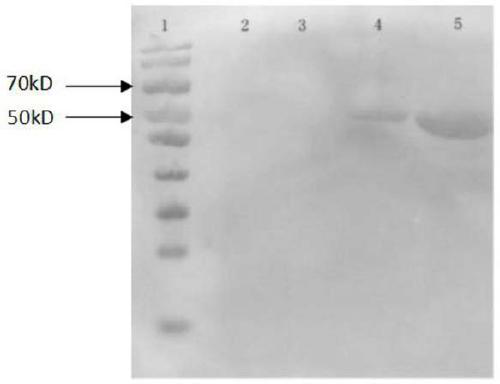
[0081] Design primers (synthesized by Shanghai Sangon) VP2-U (sequence 1) / VP2-L (sequence 2) according to the RDPV LN strain gene sequence preserved by our company, and use PCR to amplify the VP2 gene. The amplification results are as follows figure 1 As shown, the amplified fragment is the same size as the target fragment.
[0082] VP2-U: 5'GGATCCACCATGGGTGATGGAG 3' (SEQ ID NO: 1)
[0083]VP2-L: 5' CCGCTCGAGTCAATATAATTTTCTAGG 3' (SEQ ID NO: 2)
[0084] The PCR amplification conditions are:
[0085]
[0086] It can be seen from the figure that the size of the amplified fragment is consistent with that of the VP2 gene.
[0087] 2. Construction of a shuttle vector containing the VP2 gene of the raccoon parvovirus (RDPV) structural protein
[0088] The PCR products obtained above were double-digested with restriction endonuclea...
Embodiment 2
[0147] Embodiment 2 produces the preparation and preservation of seed virus batch
[0148] The recombinant baculovirus preparation process mainly includes recombinant baculovirus (P1 virus). The seed poison for production is to add 10% fetal bovine serum to the first generation poison and store in -80°C. The seed virus batch for production is usually prepared by infecting sf9 cells with subpackaged and preserved P1 virus to prepare P2 virus, and so on to prepare P3 virus.
[0149] Example 3 Vaccine Production
[0150] 1. Resuscitate 1ml of the seed cells of frozen sf9 insect cells in 100ml of serum-free medium, place them in a 500ml screw-top glass Erlenmeyer shaker flask for culture, the temperature of the shaker is 27°C, and the speed is 110r / min; after 48 hours of culture, add 200ml of serum-free medium Serum culture medium, diluted and passaged in a 3000ml screw-top glass Erlenmeyer flask; after 48 hours of culture, add 400ml of serum-free medium; every 48 hours, 1:2 dil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com