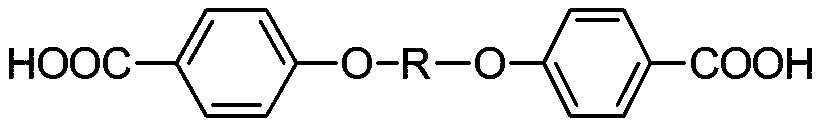Aromatic diether dicarboxylic acid and preparation method thereof
An aromatic and dicarboxylic acid technology, which is applied in the preparation of carboxylic acid nitrile, nitrile preparation, chemical instruments and methods, etc., can solve the problems of tight supply, high content of p-carboxybenzaldehyde, etc., and achieve increased yield, effective collision probability, and The effect of alleviating the tight supply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Synthesis of nitrile compounds
[0034] Under the protection of nitrogen at room temperature, add 45.6g of bisphenol A (0.2mol) and 41.4g of finely ground anhydrous K 2 CO 3 (0.3mol), 60.5g p-chlorobenzonitrile (0.44mol) and 200ml NMP, 50ml toluene, heat up to 150°C and reflux to divide the water, wait for the anhydrous to come out and finish the water separation, let off the water and most of the toluene after the water separation is completed, Stir and heat up to 180°C. After about 5 hours of reaction, the reaction is over. After cooling, pour the product into 200ml of high-speed stirring water, filter, wash the solid with water, and dry to obtain bisphenol A diether dicarbonitrile.
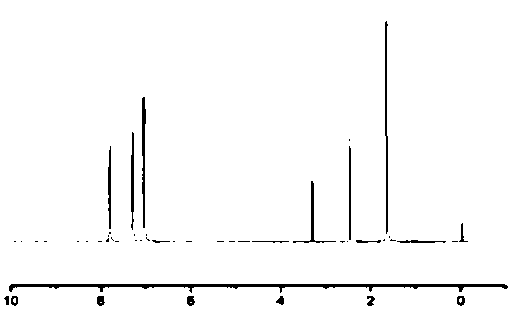
[0035] The result of NMR analysis of the product was 1H-NMR (DMSO-d6 (deuterated dimethyl sulfoxide), δ, ppm) 7.79 (4H, d), 7.29 (4H, d), 1.65 (6H, d).
[0036] The resulting bisphenol A diether dinitrile has a melting point of 124-125° C. as measured by a hot stage polarizing micr...
Embodiment 2
[0041] (1) Synthesis of nitrile compounds
[0042] Under the protection of nitrogen at room temperature, add 74.484g biphenol (0.4mol) and 52.95g finely ground anhydrous sodium carbonate (0.5mol ), 121g p-chlorobenzonitrile (0.88mol) and 800ml sulfolane, 100ml toluene, heat up to 160°C to reflux for water separation, wait for anhydrous to come out and finish water separation, let off water and most of toluene after water separation, stir and heat up to 200 ℃, after about 3 hours of reaction, the reaction is over. After cooling, pour the product into 400ml of high-speed stirred water, filter, wash the solid with water, and dry to obtain biphenol-type diether dicarbonitrile.
[0043] The resulting biphenol type diether dinitrile has a melting point of 238-240° C. as measured by a hot stage polarizing microscope.
[0044] (2) Synthesis of acid compounds
[0045] Add 21.5g of biphenol-type diether dinitrile (0.05mol), 28g of KOH (0.5mol), 200ml of DMSO and 50ml of water into a 5...
Embodiment 3
[0048] (1) Synthesis of nitrile compounds
[0049] Under the protection of nitrogen at room temperature, add 75.27g 6,6'-dihydroxy-2,2'-bipyridine (0.4mol) to a 1000ml four-neck flask equipped with mechanical stirring, water separator, nitrogen inlet and thermometer, 52.95 g finely ground anhydrous sodium carbonate (0.5mol), 121g p-chlorobenzonitrile (0.88mol) and 800ml sulfolane, 100ml toluene, heat up to 160°C and reflux to divide the water. Remove water and most of the toluene, stir and heat up to 200°C, react for about 3 hours, and the reaction is over. After cooling, pour the product into 400ml of high-speed stirring water, filter, wash the solid, and dry to obtain 6,6'-dihydroxy -2,2'-Bipyridyl Diether Dicarbonitrile.
[0050]The obtained 6,6'-dihydroxy-2,2'-bipyridyl diether dinitrile has a melting point of 262-265°C as measured by a hot stage polarizing microscope.
[0051] (2) Synthesis of acid compounds
[0052] Add 21.6g 6,6'-dihydroxy-2,2'-bipyridine diphenol ty...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com