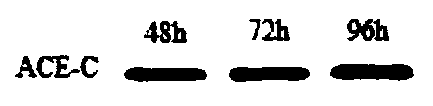Construction and application of novel angiotensin converting enzyme inhibitor screening model
A technology for screening angiotensin and inhibitors, applied in biochemical equipment and methods, enzymes, enzymes, etc., can solve the problems of ignoring the specificity of ACE membrane protein and the difference in activity of ACE inhibitory peptides, etc., to achieve stable integration and improve purity , a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Construction of the C domain recombinant plasmid of ACE
[0027] Step 1. According to the full gene information of ACE in the NCBI database (sequence number: 1636), use the HG11598-NH plasmid containing complete human ACEORF as a template, and use PrimerPremier5.0 to design primers, and obtain the upstream primer as: 5'AAGCTTATGCCGTTGCCTGACAACTACCC3' (containing HindⅢ restriction site), the downstream primer is 5'GAATTCTCACACCATCACCATCACCATGGAGTGTCTCAGGCTCCACCTC3' (containing EcoRI restriction site and histidine tag sequence). Use TaqDNA polymerase to amplify through a PCR amplification instrument. After the reaction is over, use agarose gel electrophoresis to test the PCR product, and recover the product with the correct test result. The recovered product is the target gene, that is, the human source. Angiotensin converting enzyme C domain protein cDNA.
[0028] Step 2, the gene fragment of the C domain protein of human source ACE and the eukaryotic express...
Embodiment 2
[0030] Example 2: Expression and purification of the C domain protein of ACE
[0031] Step 1, in F12-K (Gibco) medium containing 10% fetal bovine serum, 37 ° C, 5% CO 2 CHO-K1 cells were cultured under these conditions. 2 × 10 per well in a 6-well plate 5 Cells were plated, and after overnight culture, transfected with transfection reagent TurboFect for 1 to 5 hours; the transfection amount of plasmid pcDNA3.1(+)-ACEC was 500 to 900 ng per well, and the amount of transfection reagent TurboFect was 1 to 500 ng. 5 μL.
[0032] Step 2: Cultivate the transfected cells, recover the cells cultured for 48h, 72h, and 96h respectively, and use the cell membrane protein and cytoplasmic protein extraction kit to separate the C domain protein of ACE from the cell membrane to obtain the crude membrane The protein concentration was 23 μg / μL, and Western Blot was used to identify whether the protein structure and the expression of the His-tag tag were correct for the isolated membrane pro...
Embodiment 3
[0034] Example 3: Construction of a screening model for novel angiotensin-converting enzyme inhibitors
[0035] Step 1. Dissolve phospholipid / cholesterol in a mixed solution of chloroform and methanol with a mixing ratio of 1:1 to 4:1 according to the ratio of 6:1 to 9:1, and remove chloroform and methanol by rotary evaporation at 50 to 70°C. Methanol was added to the reconstitution buffer (10-40mM HEPES, 100-150mM NaCl, 2-6% w / w glycerol, pH7.5) to dissolve the liposomes. Extruded from the extruder, finally obtained a simulated film that was stable for 4 hours at room temperature.
[0036] Step 2. On this basis, reconstitute the purified C-domain protein of ACE and the simulated membrane prepared in Step 1 at a ratio of 1:10 to 1:30, and control the reaction temperature to 20-37°C. The time ranges from 10 to 40 minutes. During this process, the mixed system is slightly shaken. After the band reaction, a screening model for a new type of angiotensin-converting enzyme inhibito...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com