A kind of stable polypeptide inhibitor targeting hdac and use thereof
A technology of polypeptides and inhibitors, applied in the field of bioengineering, can solve problems such as large toxic side effects and poor effects, and achieve the effects of inhibiting proliferation, solving toxic and side effects, and improving toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Design of Polypeptide Inhibitors
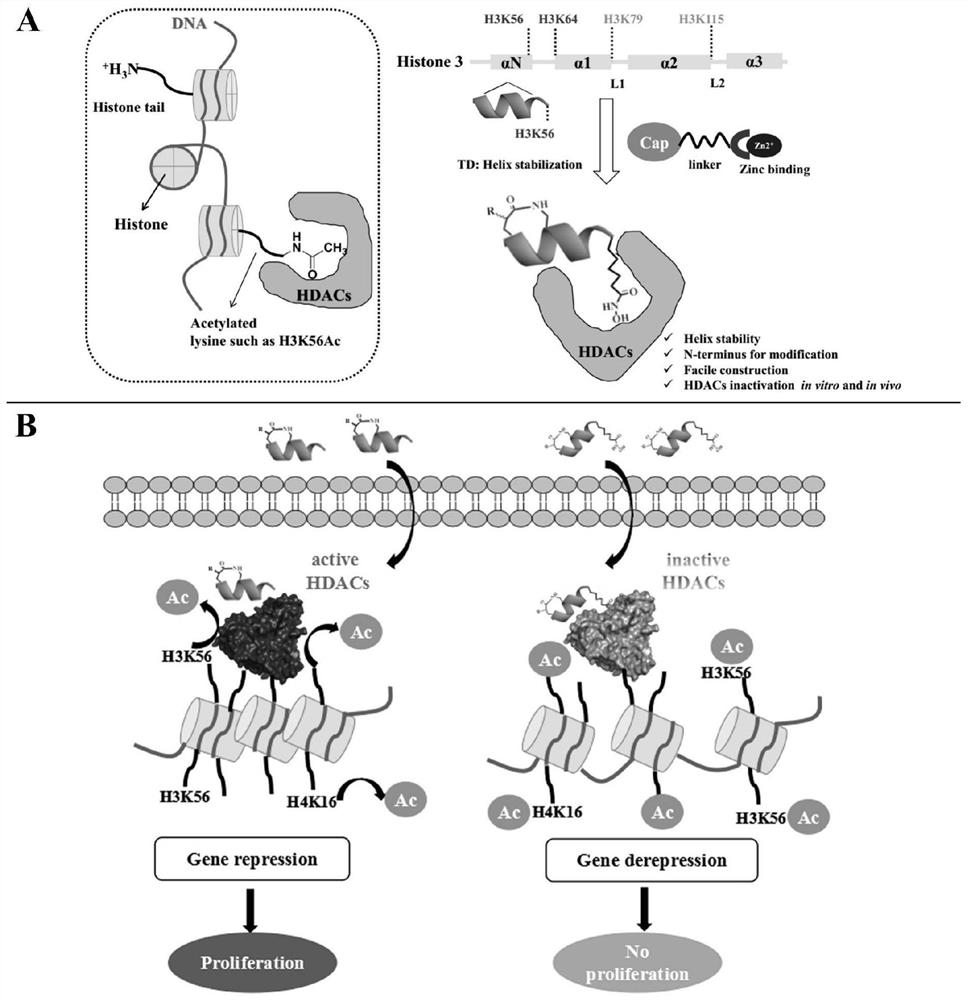
[0042] The present invention adopts the way of polypeptide-small molecule covalent coupling to design HDAC polypeptide inhibitor, such as figure 1 As shown, we selected a polypeptide sequence (TVALREIRRYQK(Ac)) near the H3K56 (the 56th amino acid lysine side chain amino group of histone H3 was modified by acetylation), a selective substrate of HDAC1, because K56 of this polypeptide is in a segment of The C-terminus of the helix is suitable for us to use the "N-terminal aspartate strategy" method to stabilize the polypeptide without affecting the recognition of the target by the C-terminal K56. In order to further improve the binding of substrate polypeptides to HDAC, we mutated the amino acid of K56 into a hydroxamic acid structure, as in figure 1 A shown.
[0043] It has been reported that this structure can chelate with the zinc ion in the catalytic region of HDAC enzyme to inhibit the activity of HDAC enzyme. The bas...
Embodiment 2
[0046] Example 2: Synthetic Preparation of Polypeptide Inhibitors
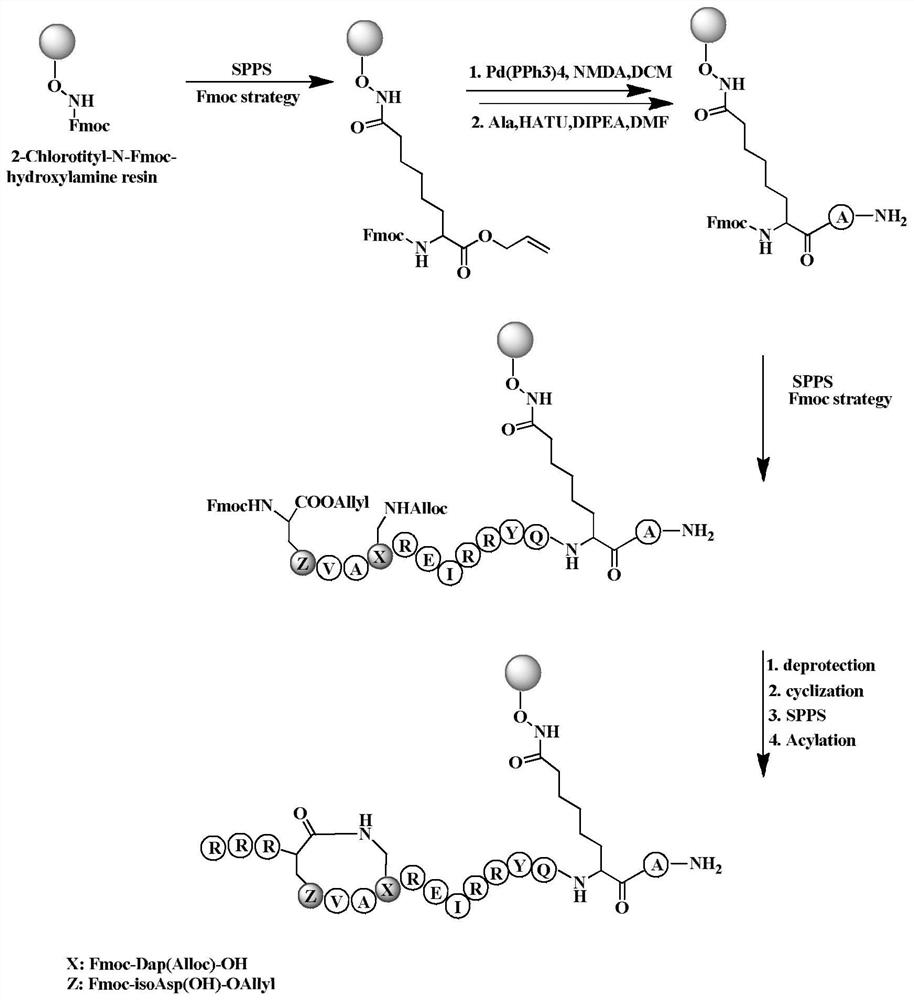
[0047] We use the internationally accepted solid-phase peptide synthesis technology (SPPS) to synthesize peptides. Its synthetic route is as figure 2 shown.
[0048] Specific steps:
[0049] (1) Synthesis of unnatural amino acid with hydroxamic acid (Fmox-Hx-OH): The synthetic route is carried out according to the method reported in the literature.
[0050] (2) Solid-phase synthesis of peptides: Weigh 100 mg of 2-Chlorotity-N-Fmoc-hydroxylamine resin into a 10 ml peptide tube, add dichloromethane (DCM), and swell with nitrogen for 10 min. 50% (v / v) morpholine in N,N-dimethylformamide (DMF) solution was added, and nitrogen was bubbled for 30min×2 to remove the Fmoc protecting group. After alternately washing the resin 6 times with DMF and DCM, the prepared (1) Fmoc-Hx-OH (5eq, 0.4M, DMF) solution, 6-chlorobenzotriazole-1,1,3,3-tetramethyl Urea hexafluorophosphate (HCTU) (5eq, 0.38M, DMF) solution, N,N-dii...
Embodiment 3
[0055] Example 3: Identification of Polypeptide Secondary Structure and Research on Inhibitory Effect on HDAC Enzyme Activity
[0056] For each peptide, circular dichroism was used to determine the conformation in water, demonstrating that the peptides stabilized in water by the method of terminal side chain-tail linking chiral diacid-modified polypeptides (e.g. Figure 5 A) and in 20% TFE solution (as Figure 5 B) has a stable α-helical conformation, in which TFE (trifluoroethanol) has the effect of promoting the helix. To identify the inhibitory effect of HDAC enzyme activity, use a commercially purchased HDAC enzyme activity detection kit for operation, and each operation is repeated at least three times. The enzyme activity results are as follows: Image 6 B (design of polypeptide inhibitory library and screening of the enzymatic activity inhibitory effect of HeLa nuclear extract) and Image 6 C( Image 6 C: The inhibitory effect of different polypeptide inhibitors on t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com