Stable polypeptide inhibitor targeting HDAC and application of stable polypeptide inhibitor
A technology of polypeptides and inhibitors, applied in the field of bioengineering, can solve the problems of large toxic and side effects and poor effects, and achieve the effects of inhibiting proliferation, solving toxic and side effects, and improving the ability of cells to penetrate membranes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the design of polypeptide inhibitor
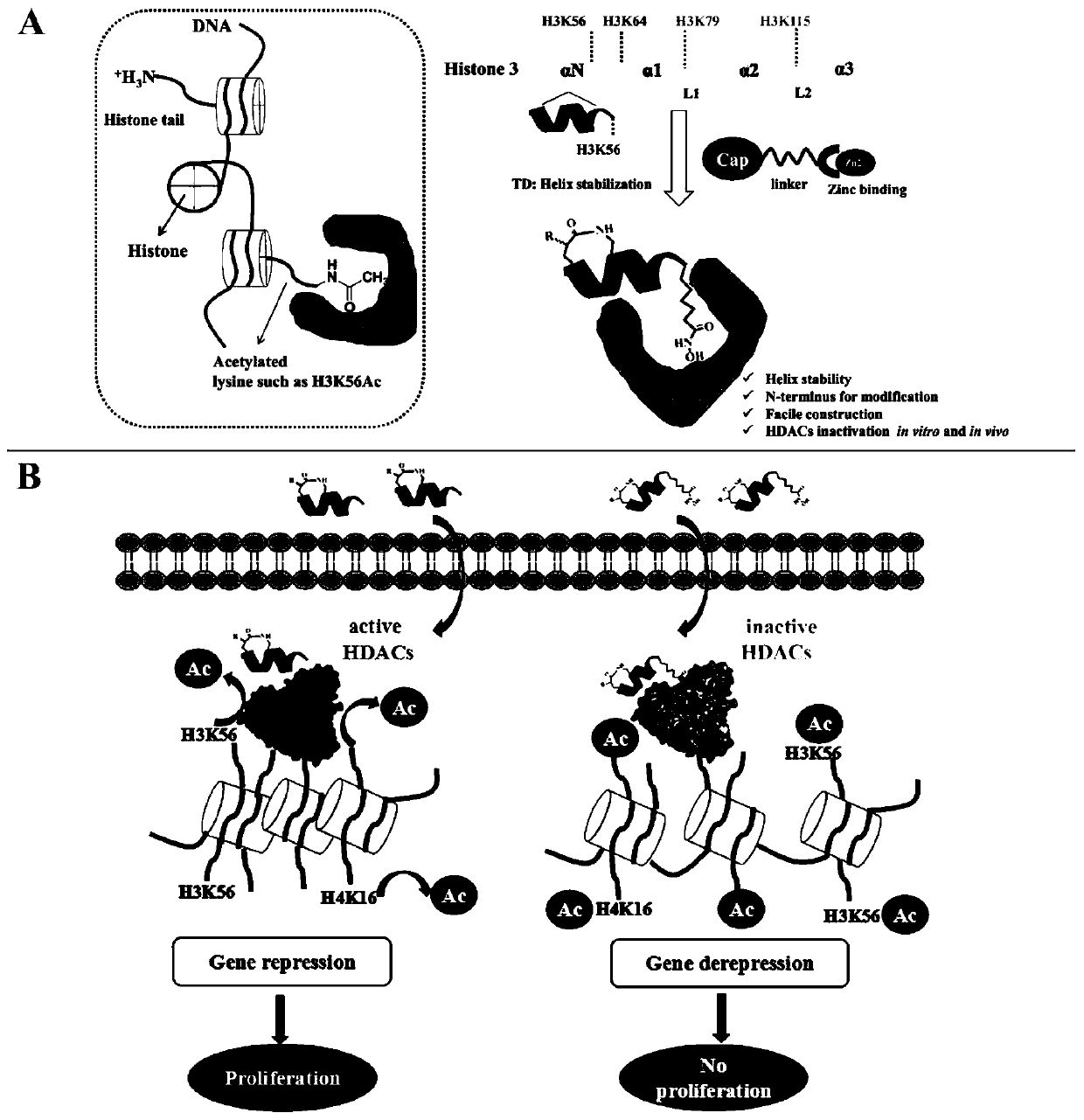
[0042] The present invention adopts the way of polypeptide-small molecule covalent coupling to design HDAC polypeptide inhibitors, such as figure 1 As shown, we selected a polypeptide sequence (TVALREIRRYQK(Ac)) near the HDAC1 selective substrate H3K56 (the 56th amino acid lysine side chain amino of histone H3 was modified by acetylation), because the K56 of this polypeptide is in a The C-terminus of the helix is suitable for us to use the "N-terminal aspartic acid strategy" method to stabilize the polypeptide without affecting the recognition of the target by the C-terminal K56. In order to further improve the binding of the substrate polypeptide to HDAC, we mutated the amino acid of K56 into a hydroxamic acid structure, such as figure 1 As shown in A.
[0043] This structure was reported to be able to chelate zinc ions in the catalytic region of HDAC enzymes to inhibit HDAC enzyme activity. The basic structure o...
Embodiment 2
[0046] Embodiment 2: the synthetic preparation of polypeptide inhibitor
[0047] We use the internationally accepted solid-phase peptide synthesis technique (SPPS) to synthesize peptides. Its synthetic route is as follows figure 2 shown.
[0048] Specific steps:
[0049] (1) Synthesis of unnatural amino acid (Fmox-Hx-OH) with hydroxamic acid: the synthetic route is completely carried out according to the method reported in the literature.
[0050] (2) Solid-phase synthesis of polypeptides: Weigh 100 mg of 2-Chlorotity-N-Fmoc-hydroxylamine resin into a 10 ml peptide tube, add dichloromethane (DCM), and swell with nitrogen gas for 10 min. Add 50% (v / v) morpholine in N,N-dimethylformamide (DMF) solution, blow nitrogen gas for 30min×2, and remove the Fmoc protecting group. After washing the resin 6 times alternately with DMF and DCM, the prepared (1) Fmoc-Hx-OH (5eq, 0.4M, DMF) solution, 6-chlorobenzotriazole-1,1,3,3-tetramethyl Urea hexafluorophosphate (HCTU) (5eq, 0.38M, D...
Embodiment 3
[0055] Example 3: Identification of the secondary structure of the polypeptide and its inhibitory effect on HDAC enzyme activity
[0056] For each peptide, conformation in water was determined by circular dichroism chromatography, demonstrating that peptides stabilized by terminal side chain-to-tail linkage of chiral diacid-modified peptides in water (e.g. Figure 5 A) and in 20% TFE solution (such as Figure 5 B) has a stable α-helical conformation, in which TFE (trifluoroethanol) has the effect of promoting the helix. The identification of HDAC enzyme activity inhibitory effect is carried out with commercially purchased HDAC enzyme activity detection kits, and each operation is repeated at least three times. The enzyme activity results are as follows: Figure 6 B (the design of peptide inhibition library and the screening of the enzyme activity inhibitory effect of HeLa nucleus extract) and Figure 6 C( Figure 6 C: the inhibitory effect of different polypeptide inhibitor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com