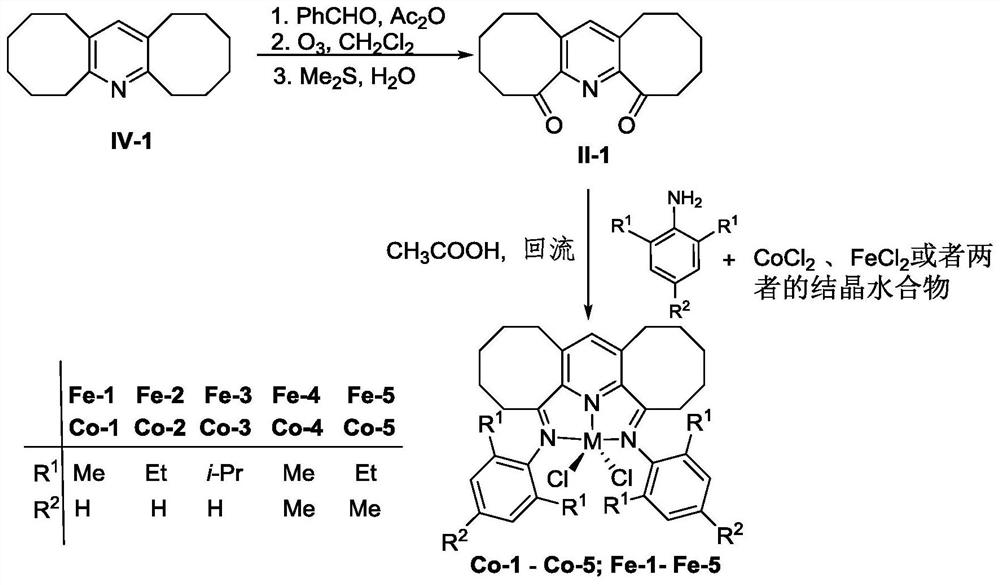
Diaryl imine pyridyl complexes containing flexible eight-membered rings and their preparation methods and applications
A kind of compound and composition technology, applied in the field of diarylimine pyridyl complex and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1 Preparation of intermediate α,α'-dioxo-2,3:5,6-bis(hexamethylene)pyridine compound shown in formula II-1
[0100]
[0101] 24.30g (0.10mol) 2,3:5,6-two (hexamethylene) pyridine compound shown in IV-1 formula, 106.00g (1.00mol) benzaldehyde and 81.60g (0.80mol) acetic anhydride in Under nitrogen atmosphere, react at 180°C for 72h. After the reaction was detected by TLC, it was cooled to room temperature, and a vacuum distillation device was set up, and the reaction solution was subjected to vacuum distillation (100° C., 10 mm Hg) to remove unreacted benzaldehyde, acetic anhydride, and by-products. The crude product obtained after distillation under reduced pressure was dissolved in 500 mL of dichloromethane. The reaction solution was cooled to below -40°C and passed through dry O 3 / O2 , carry out ozonation reaction until the solution turns light yellow, react for about 3 hours, TLC detects that the reaction is complete, stop passing O 3 / O 2 , N 2 Bubb...
Embodiment 2
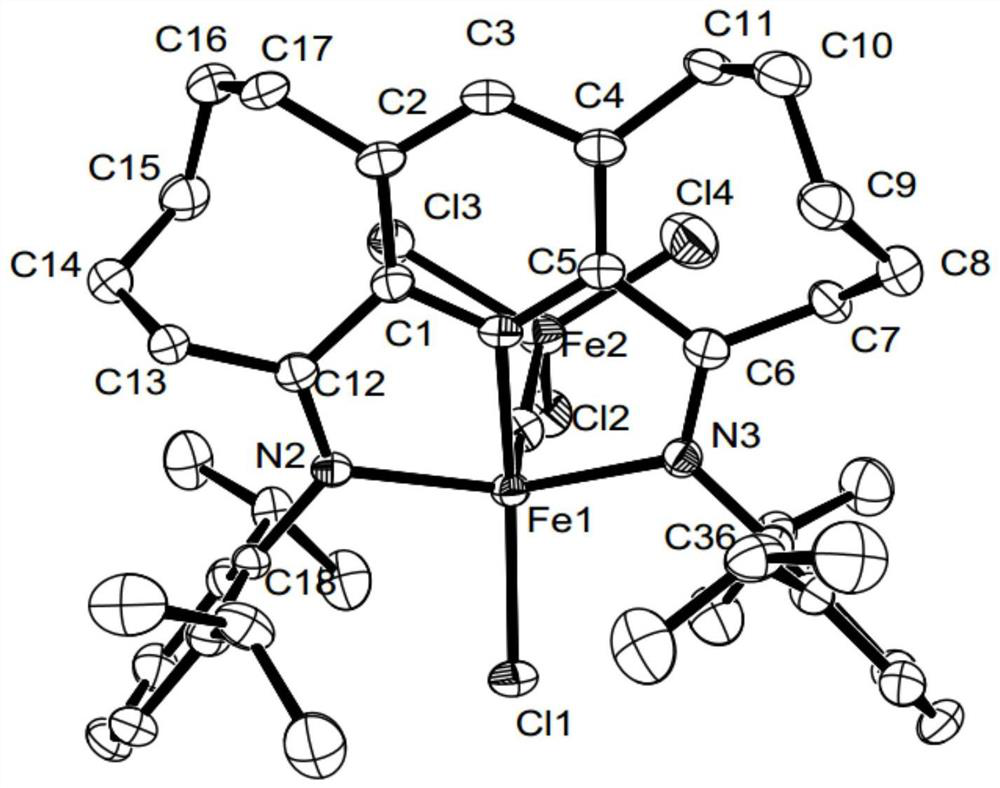
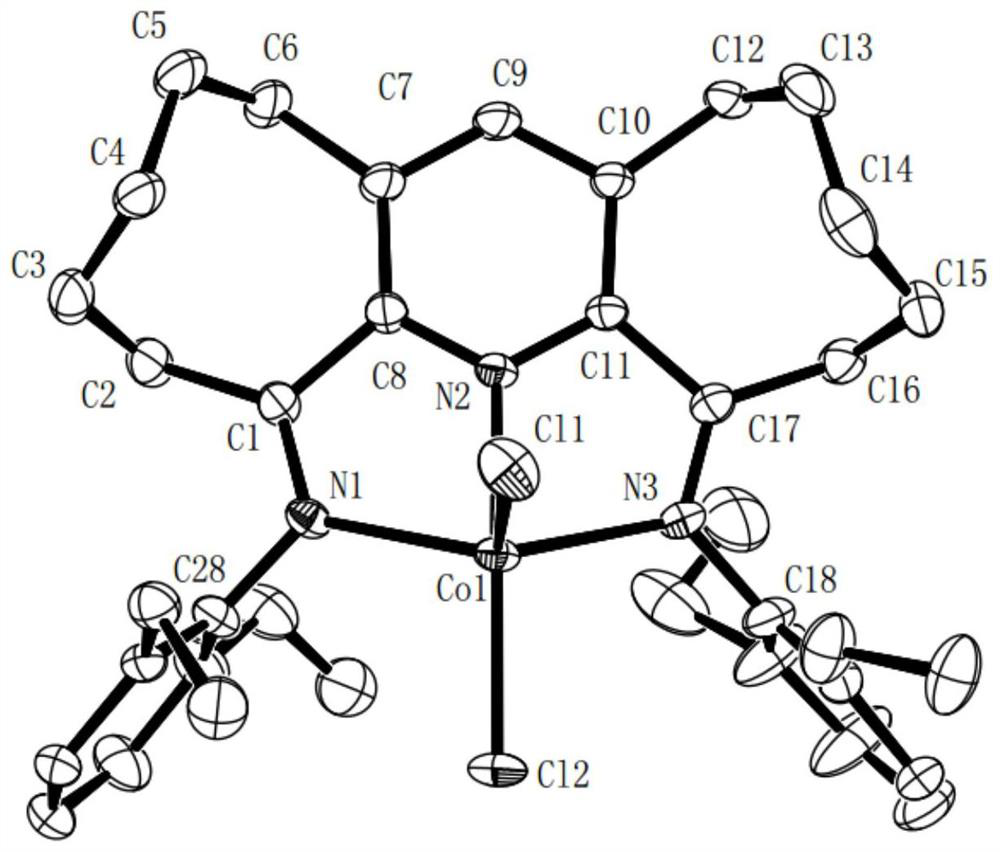
[0106] Example 2 Preparation of α,α'-bis(2,6-dimethylphenylimino)-2,3:5,6-bis(hexamethylene)pyridylferric chloride represented by the following formula [Fe-1](R 1 is methyl, R 2 for hydrogen)
[0107]
[0108] 0.27g (1.0mmol) α,α'-dioxo-2,3:5,6-bis(hexamethylene)pyridine compound represented by formula II-1, 0.48g (4.0mmol) 2,6- Dimethylaniline and 0.19g (0.9mol) FeCl 2 4H 2 O was dissolved in 15 mL of acetic acid, stirred and refluxed at 130°C for 12 hours under nitrogen atmosphere, the reaction liquid was concentrated, a large amount of ether was added to precipitate, the precipitate was collected by filtration, and then the precipitated substrate was dissolved in methanol, the solution was concentrated, and a large amount of Diethyl ether precipitated and the precipitate was collected by filtration and washed with copious amounts of diethyl ether. A light blue powder (0.49 g, 89%) was obtained.
[0109] The structural confirmation data are as follows:
[0110] FT-...
Embodiment 3
[0112] Example 3 Preparation of α,α'-bis(2,6-diethylphenylimino)-2,3:5,6-bis(hexamethylene)pyridylferric chloride represented by the following formula [Fe-2](R 1 is ethyl, R 2 for hydrogen)
[0113]
[0114] 0.27g (1.0mmol) α,α'-dioxo-2,3:5,6-bis(hexamethylene)pyridine compound represented by formula II-1, 0.59g (4.0mmol) 2,6- Diethylaniline and 0.19g (0.9mol) FeCl 2 4H 2 O was dissolved in 15 mL of acetic acid, stirred and refluxed at 130°C for 12 hours under nitrogen atmosphere, the reaction liquid was concentrated, a large amount of ether was added to precipitate, the precipitate was collected by filtration, and then the precipitated substrate was dissolved in methanol, the solution was concentrated, and a large amount of Diethyl ether precipitated and the precipitate was collected by filtration and washed with copious amounts of diethyl ether. A light blue powder (0.45 g, 75%) was obtained.
[0115] The structural confirmation data are as follows:
[0116] FT-IR ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com