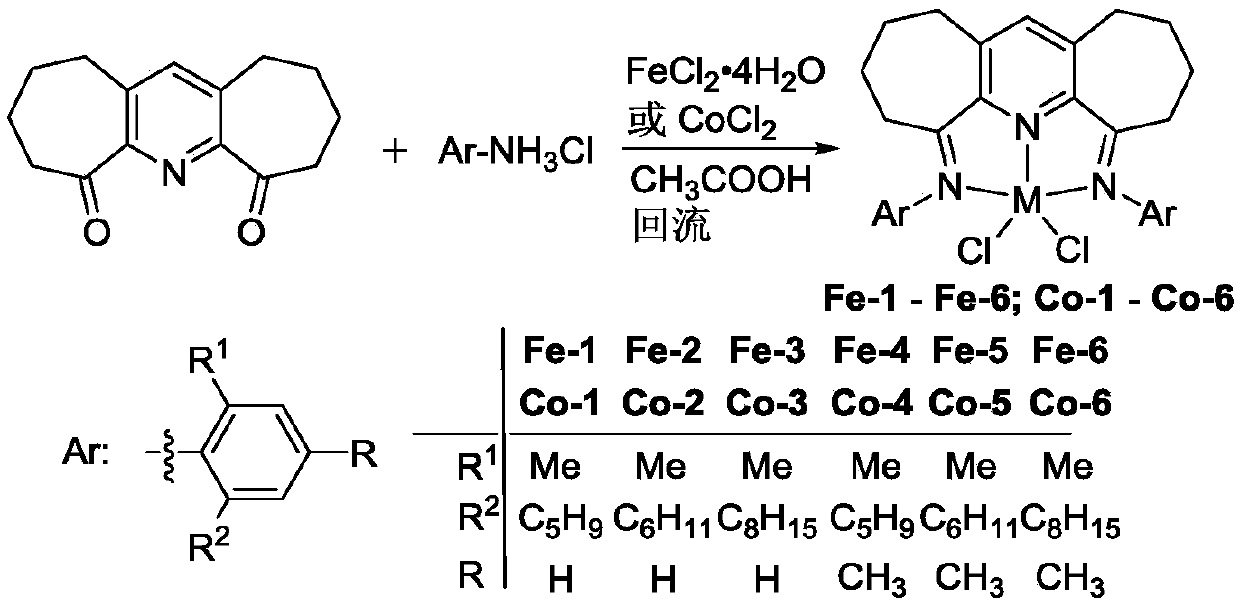
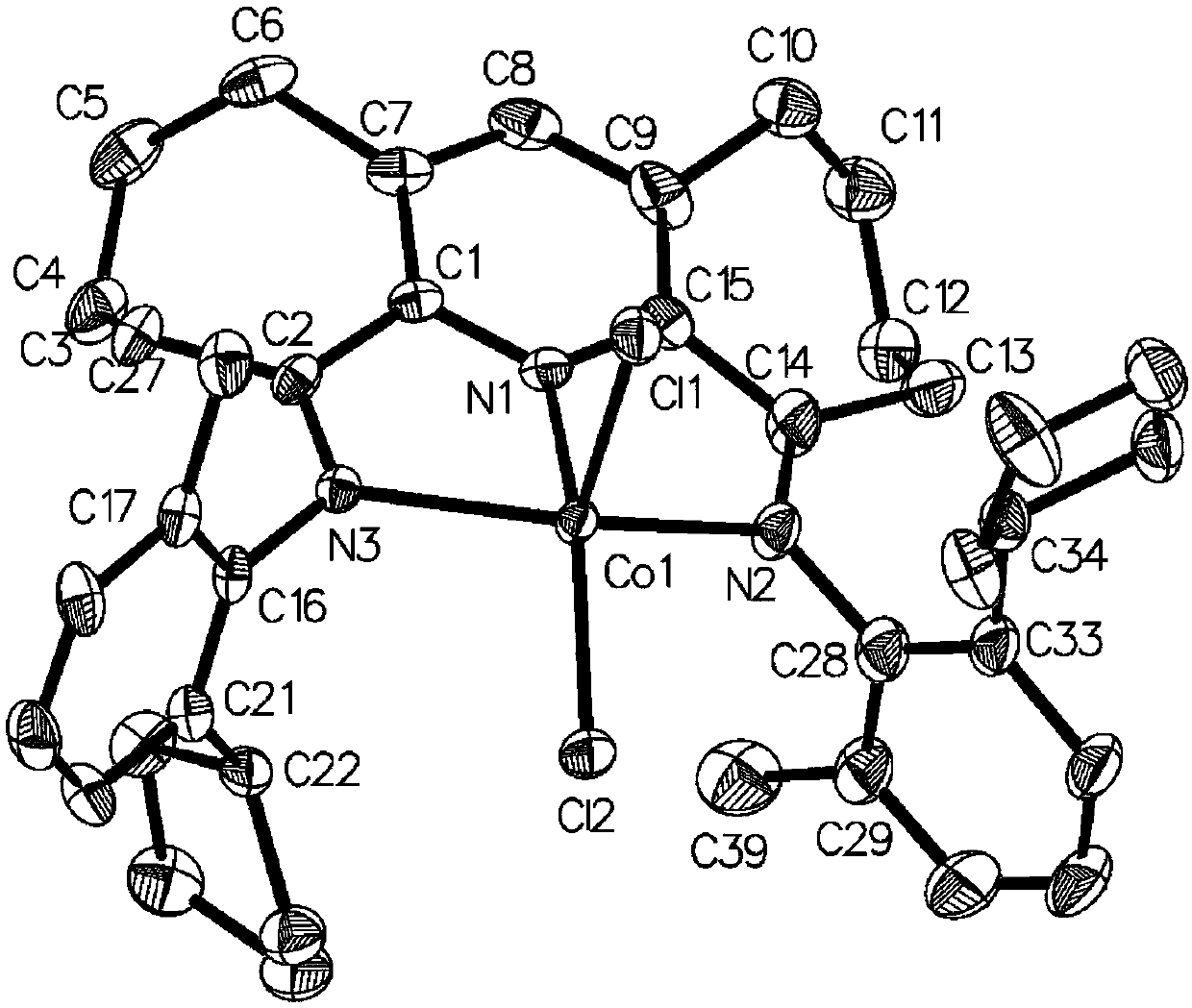
Seven-membered cyclopyridylimine complex containing large-steric-hindrance cycloalkane and preparation method and application of seven-membered cyclopyridylimine complex
A kind of compound, cycloalkyl technology, applied in the field of symmetrical seven-membered ring pyridineimino-based metal complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Example 1 Preparation of α,α'-bis(2-dimethyl-6-cyclopentylphenylimino)-2,3:5,6-bis(pentamethylene)pyridyl represented by the following formula Ferric chloride [Fe-1](R 1 is methyl, R 2 is cyclopentyl, R is hydrogen)
[0103]
[0104] 0.084g (0.2mmol) α,α'-dioxo-2,3:5,6-bis(pentamethylene)pyridine compound represented by formula (II-1), 0.048g (0.4mmol) 2- Dimethyl-6-cyclopentylaniline hydrochloride and 0.035g (0.18mmol) FeCl 2 ·4H 2 O was dissolved in 10 mL of acetic acid, stirred and refluxed at 130 °C for 8 h under nitrogen atmosphere, the reaction solution was concentrated, a large amount of ether was added to precipitate, the precipitate was collected by filtration, then the precipitated substrate was dissolved in dichloromethane, the solution was concentrated, A large amount of diethyl ether was added to precipitate and the precipitate was collected by filtration and washed with a large amount of diethyl ether. After drying, a brown powder (0.046 g, 37%) wa...
Embodiment 2
[0108] Example 2 Preparation of α,α'-bis(2-dimethyl-6-cyclohexylphenylimino)-2,3:5,6-bis(pentamethylene)pyridylbis shown in the following formula Ferric chloride [Fe-2](R 1 is methyl, R 2 is cyclohexyl, R is hydrogen)
[0109]
[0110] 0.048g (0.2mmol) α,α'-dioxo-2,3:5,6-bis(pentamethylene)pyridine compound represented by formula (II-1), 0.09g (0.4mmol) 2- Dimethyl-6-cyclohexylaniline hydrochloride and 0.035g (0.18mmol) FeCl 2 ·4H 2 O was dissolved in 10 mL of acetic acid, stirred and refluxed at 130 °C for 8 h under a nitrogen atmosphere, the reaction solution was concentrated, a large amount of ether was added to precipitate, the precipitate was collected by filtration, then the precipitated substrate was dissolved in methanol, the solution was concentrated, and a large amount of Diethyl ether precipitated and the precipitate was collected by filtration and washed with copious amounts of diethyl ether. After drying a brown powder (0.07 g, 54%) was obtained.
[0111]...
Embodiment 3
[0114] Example 3 Preparation of α,α'-bis(2-dimethyl-6-cyclooctylphenylimino)-2,3:5,6-bis(pentamethylene)pyridyl shown in the following formula Ferric chloride [Fe-3](R 1 is methyl, R 2 is cyclooctyl, R is hydrogen)
[0115]
[0116] 0.048g (0.2mmol) α,α'-dioxo-2,3:5,6-bis(pentamethylene)pyridine compound represented by formula (II-1), 0.10g (0.4mmol) 2- Dimethyl-6-cyclooctylaniline hydrochloride and 0.035g (0.18mmol) FeCl 2 ·4H 2 O was dissolved in 10 mL of acetic acid, stirred and refluxed at 130 °C for 8 h under a nitrogen atmosphere, the reaction solution was concentrated, a large amount of ether was added to precipitate, the precipitate was collected by filtration, then the precipitated substrate was dissolved in methanol, the solution was concentrated, and a large amount of Diethyl ether precipitated and the precipitate was collected by filtration and washed with copious amounts of diethyl ether. After drying a brown powder (0.01 g, 11%) was obtained.
[0117] Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com