A kind of purification method of valrubicin
A technology of valrubicin and a purification method, which is applied in the field of purification of valrubicin, can solve problems such as unclear single impurity content, high single impurity content, and large environmental pollution, and achieve simple and easy process, good effect, The effect of little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
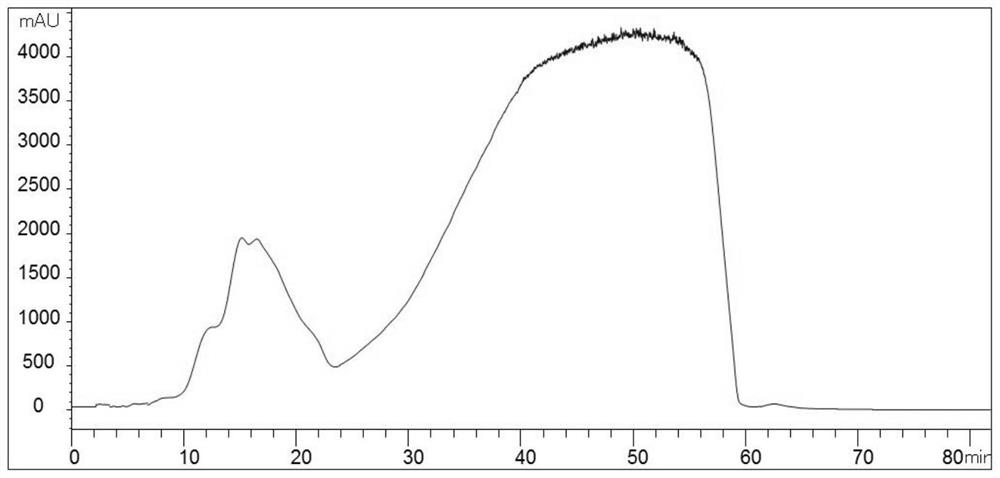
[0044] Embodiment 1 valrubicin crude product purification
[0045] Chromatographic column: valrubicin preparative column (100mm x 250mm) (the filler used is silica gel bonded with octadecylsilane and divinylbenzene)
[0046] mobile phase:
[0047] A: 0.1% formic acid aqueous solution B: Methanol (containing 0.1% formic acid)
[0048] Flow rate: 400ml / min Injection volume: 400ml Column temperature: room temperature
[0049]Sample: 100mg / ml Pressure: 1.2M Pa
[0050] Instrument: Sepax preparative chromatograph
[0051] Detection wavelength: UV@254nm
[0052] Isocratic elution: 75% B
[0053] The specific method is as follows:
[0054] 1. Sample weighing: weigh 40 g (4.8% load) of valrubicin crude product on an analytical balance;
[0055] 2. Solvent preparation: prepare 400ml of 75% methanol aqueous solution containing 0.1% formic acid, measure 328ml of methanol, 72ml of purified water, and measure 400ul of formic acid with a pipette gun. Mix the three together and stir ...
Embodiment 2
[0095] Using the same preparative chromatograph, preparative column and elution method as in Example 1
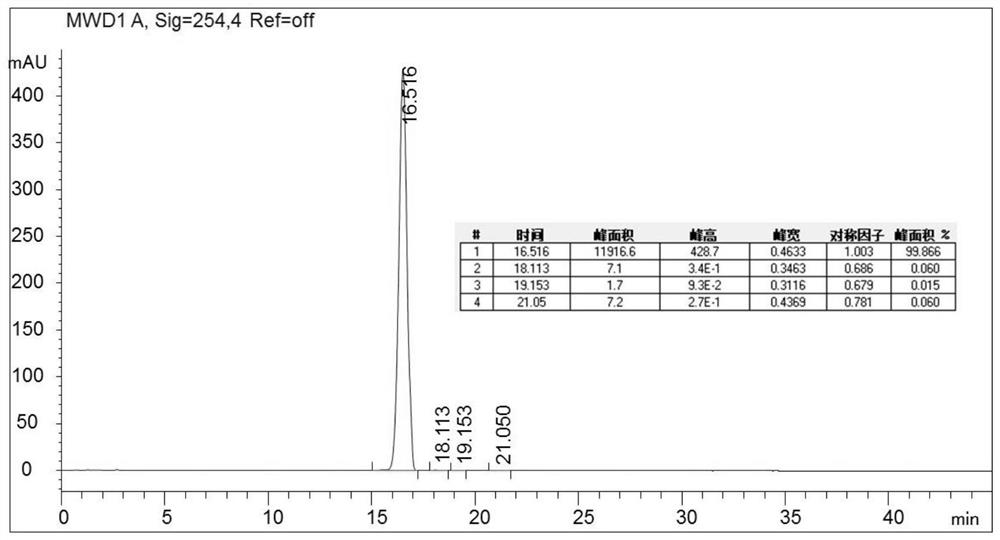
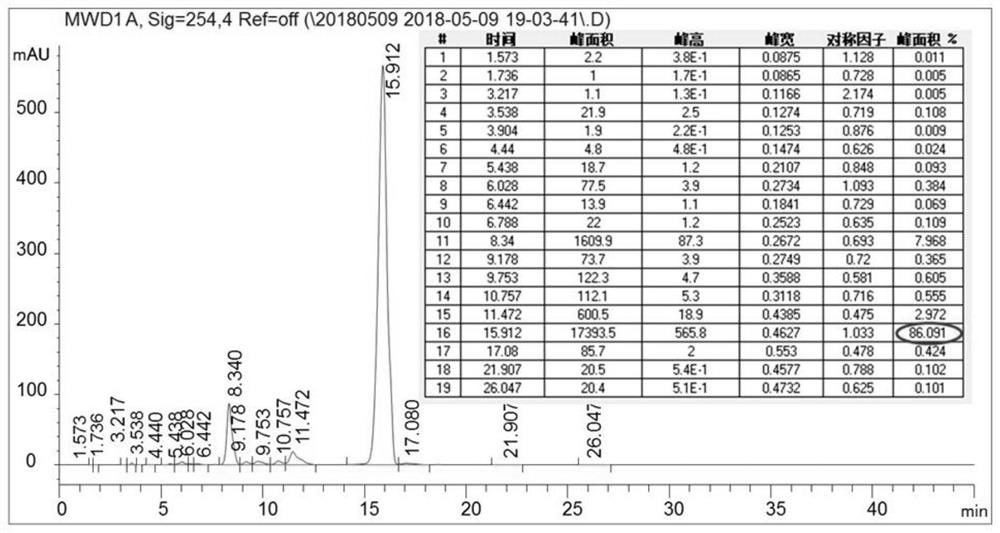
[0096] Get content 86.09% (m / m) valrubicin crude product 40g, add the 80% methanol aqueous solution (V / V) 400ml that contains 0.1% trifluoroacetic acid, stir and make fully dissolving; Go through 80% methanol (containing 0.1 % trifluoroacetic acid) aqueous solution equilibrated valrubicin chromatography column (100mm * 250mm, about 0.98kg containing filler quality), loading flow rate 200ml / min, with 80% methanol (containing 0.1% trifluoro Acetic acid) and 20% aqueous solution containing 0.1% trifluoroacetic acid elution, elution flow rate 400ml / min, elution 85min altogether, HPLC detects and collects valrubicin component solution, merges qualified components, obtains valrubicin after freeze-drying The pure product of rubicin was 26.67g, the purity detected by HPLC was 99.87%, and the recovery rate was 66.68%.
Embodiment 3
[0098] Get valrubicin crude product 85g (purity 96.5%), add the aqueous solution 800ml that contains the 80% methyl alcohol (V / V) of 0.1% trifluoroacetic acid, stir and make fully dissolving; Trifluoroacetic acid) equilibrated valrubicin preparation column (100mm * 250mm, containing about 0.98kg of filler quality), sample loading flow rate 200ml / min, use 80% methyl alcohol (containing 0.1% trifluoroacetic acid) and 20% aqueous solution containing 0.1% trifluoroacetic acid was eluted, the elution flow rate was 400ml / min, the elution flow rate was 400ml / min, and the total elution was 85min. The valrubicin component solution was collected by HPLC detection, and the data of each component after HPLC detection As shown in Table 4:
[0099] Table 4
[0100] mass (g) Recovery rate(%) Crude 85.00 -- Purity≥99.5 76.37 89.85 Purity ≥ 99.5, and simple impurity 69.15 81.35
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com